Galnac phosphoramidites, nucleic acid conjugates thereof and their use
a technology of phosphoramidite and nucleic acid conjugates, which is applied in the field of phosphoramidite derivatives, can solve the problems of complex synthesis of available carbohydrate conjugates, and achieve the effect of easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
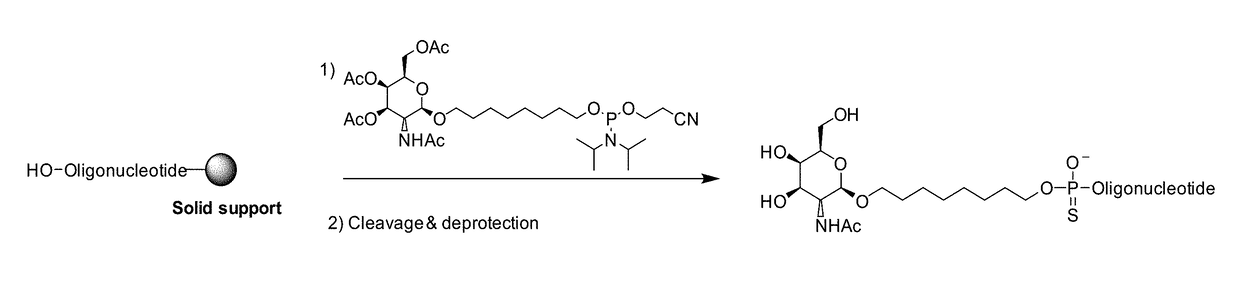
Synthesis of GalNAc Phosphoramidites
[0499]Synthesis of beta-GalNAc Phophoramidites
[0500]Step 1
[0501]tert-butyldiphenylsilylchloride (TBDPSCl) (2.6 mL, 10 mmol) was added dropwise to a mixture of ethyleneglycol (3.4 mL, 60.7 mmol) and pyridine (3.4 mL, 42.2 mmol) at room temperature. After stirring for 3.5 hours, the reaction mixture was diluted with EtOAc (50 mL). The organic phase was extracted with water (30 mL), 20% NaHCO3 (30 mL), brine (30 ml), dried over Na2SO4 and evaporated. The residue was purified by DCVC chromatography (eluent EtOAc in hexanes from 10% to 25%). Product (TBDPS glycol) was isolated as a white solid, 2.54 g, yield 84%.
[0502]Step 2
[0503]To suspension of peracetylated galactosamine (1.63 g, 4.17 mmol) in 30 mL DCM. TMSOTf (1.90 mL, 10.4 mmol) was added and reaction mixture stirred at 40-45° C. for 5 h. An additional portion of TMSOTf (0.30 mL 2.8 mmol) was added and the reaction mixture was stirred for additional 16 h. Then the reaction was quenched with NEt3 ...
example 2
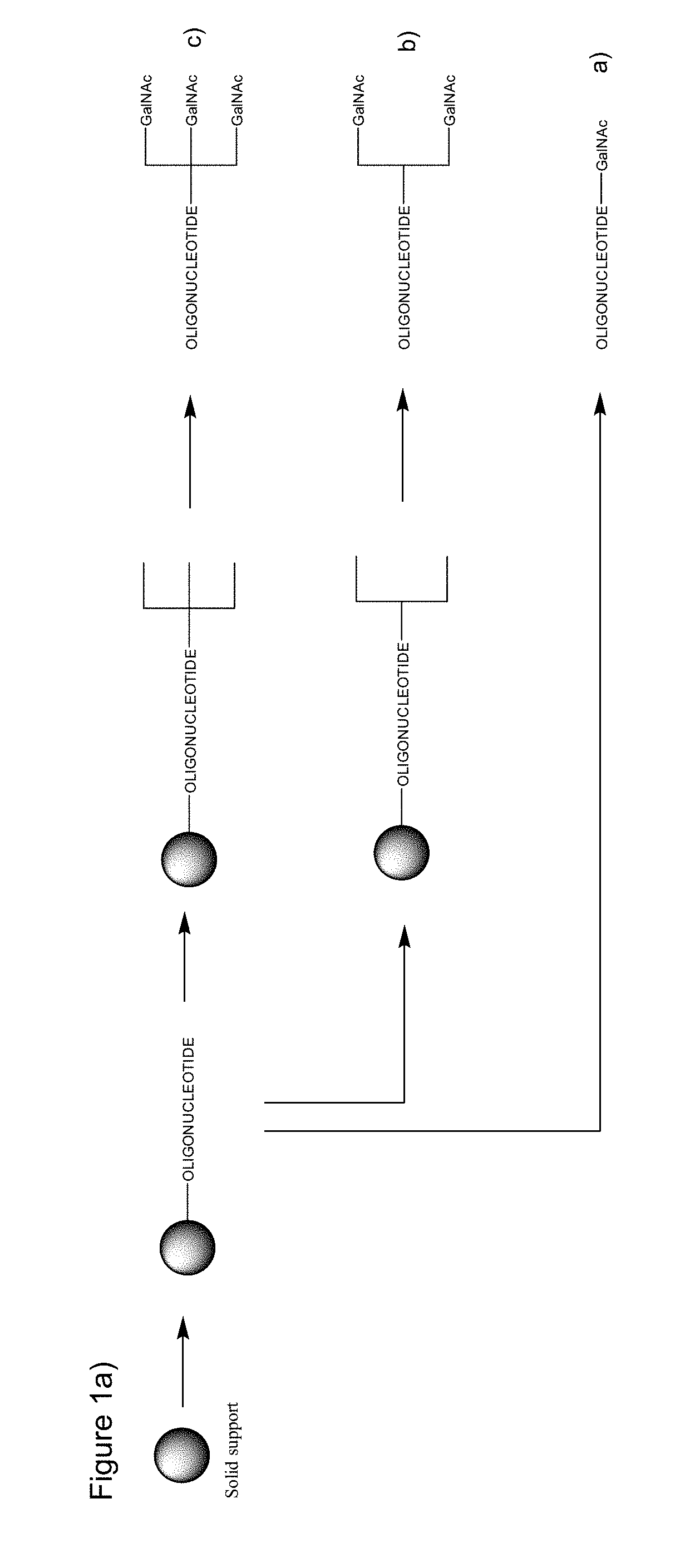
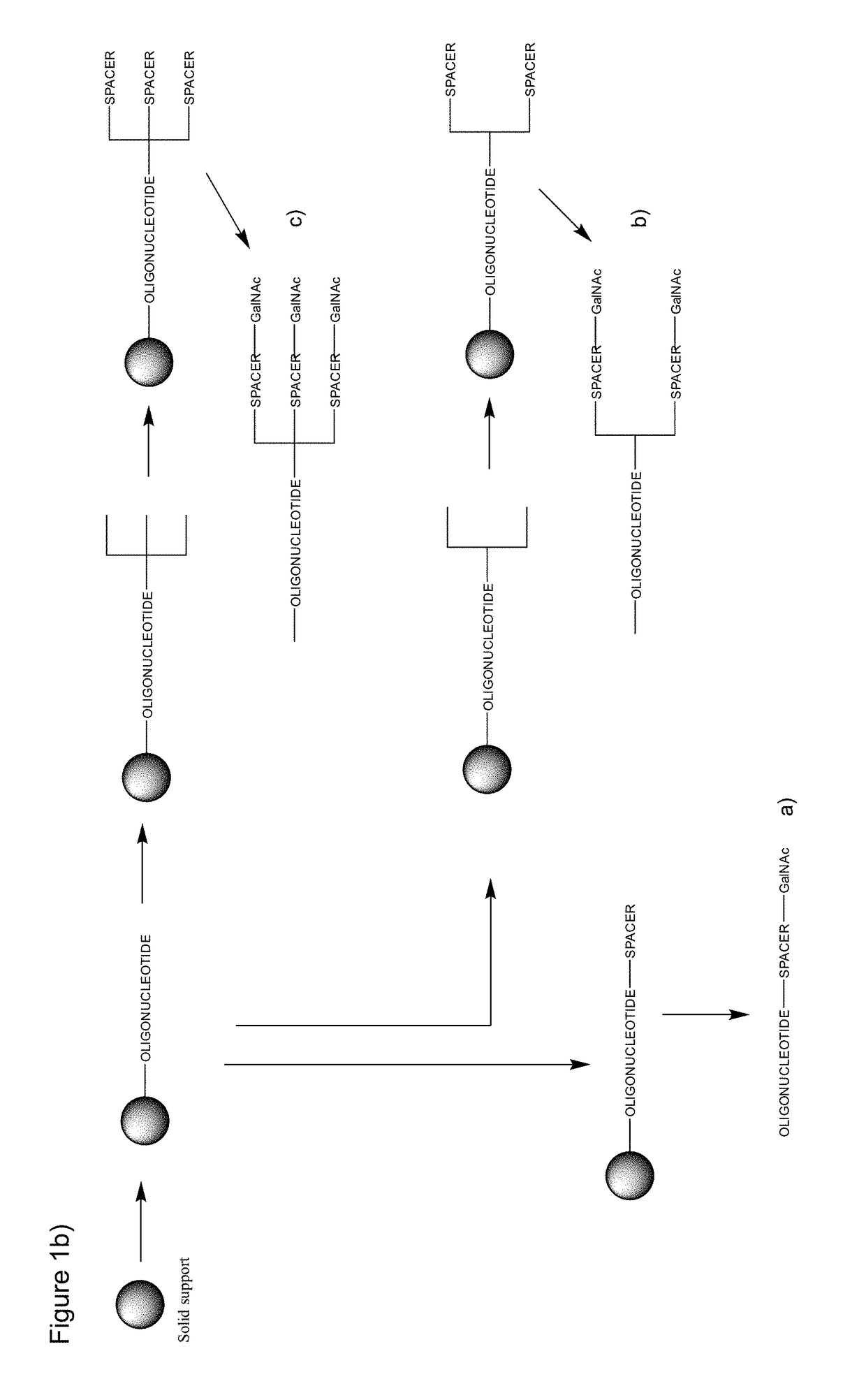
Synthesis of Oligonucleotides Using GalNAc Phosphoramidite Incorporation
[0525]Oligonucleotides were synthesized on NittoPhase Unylinker 200 supports using the phosphoramidite approach at 20 pmol scale. At the end of the synthesis, the oligonucleotides were cleaved from the solid support using concentrated ammonium hydroxide for 16 hours at 60° C. The oligonucleotides were purified by reverse phase HPLC and characterized by UPLC, and the molecular mass was further confirmed by ESI-MS.
[0526]Elongation of the Oligonucleotide:
[0527]The coupling of β-cyanoethyl-phosphoramidites (DNA-A(Bz), DNA-G(ibu), DNA-C(Bz), DNA-T, LNA-5-methyl-C(Bz), LNA-A(Bz), LNA-G(dmf), LNA-T, GalNAc phosphoramidite X and Y and spacer phosphoramidite C3 and spacer 18 (Glen Research, Sterling, Va.) was performed by using a solution of 0.1 M of phosphoramidite in acetonitrile and DCI (4,5-dicyanoimidazole) in acetonitrile (0.25 M) as activator. Thiolation for introduction of phosphorthioate linkages was carried out...
example 3
Knock Down of ApoB mRNA and Total Cholesterol with GalNAc-Conjugates in Vivo
[0532]To compare the effect of different GalNAc constructs C57BL6 / J mice were injected sc with a single dose saline or 0.25 mg / kg GalNAc2 cluster conjugated LNA-antisense oligonucleotide (GalNAc2) or equimolar amounts of LNA antisense oligonucleotides conjugated to the GalNAc constructs of the invention (FIG. 6 E, I, M and N) or unconjugated LNA antisense oligonucleotide (see Example 2, table 1 for details) and sacrificed at days 10 where liver and kidney were isolated.
[0533]Each compound was tested in an animal group containing five mice with weight of approximately 20 g. Serum samples were collected at day 3 and 7 (50 microUmouse) and at sacrifice total serum was collected for determination of total serum cholesterol as described in protocol below. The results are shown in FIG. 8.
[0534]At sacrifice RNA was isolated from liver and kidney and subjected to qPCR with ApoB specific primers and probe to analyze ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Magnetic field | aaaaa | aaaaa |
| Magnetic field | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com