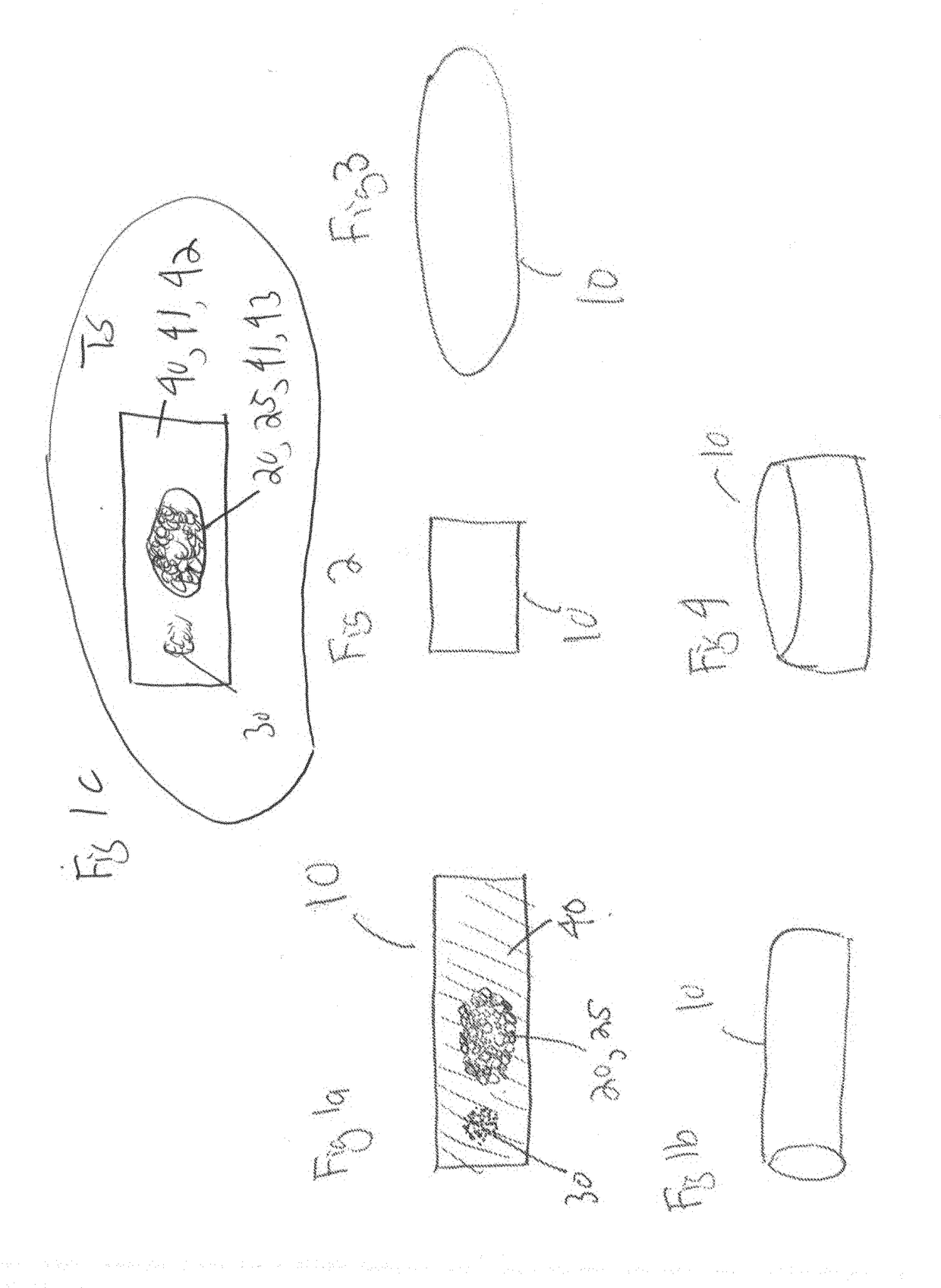
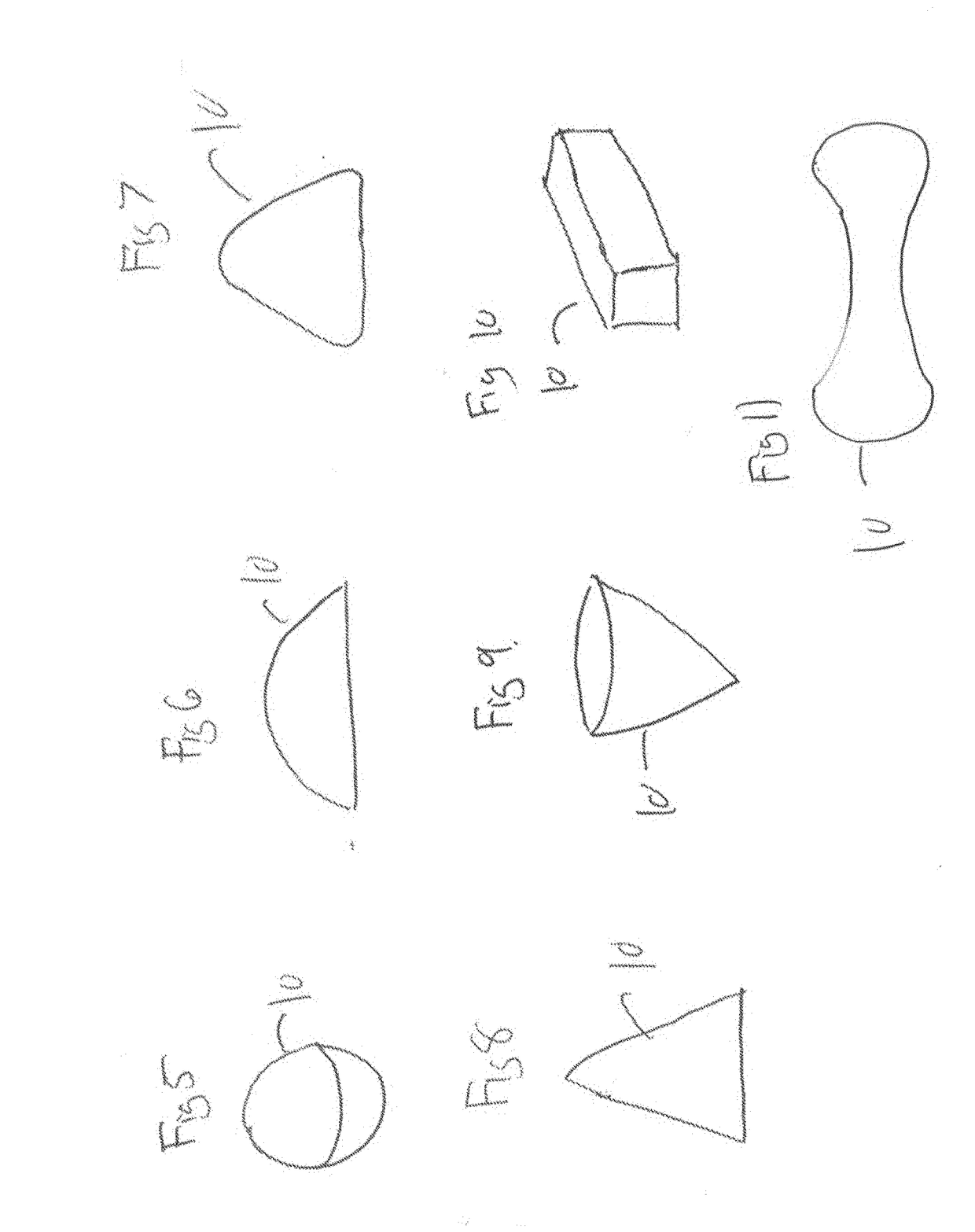
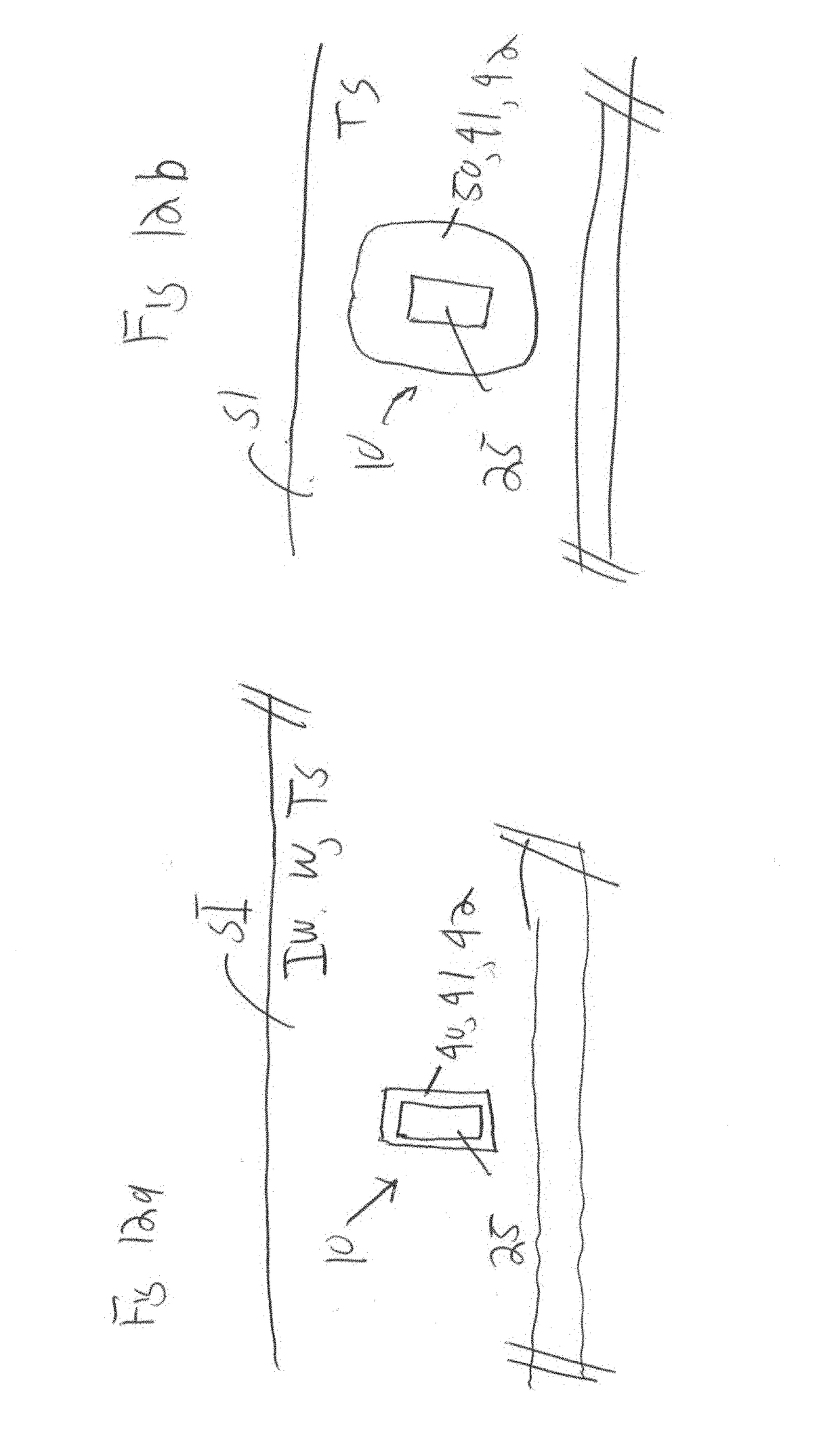
Pharmaceutical compositions and methods for fabrication of solid masses comprising polypeptides and/or proteins
a technology of polypeptides and compositions, applied in the direction of peptide/protein ingredients, metabolism disorders, antibody medical ingredients, etc., can solve the problems of loss of bioactivity of drugs, protein denaturation, limited application, etc., to improve the bioactivity of drugs, improve the yield of drugs, and reduce protein denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Micro-Tablets Comprising Human IG and PEG
[0112]Materials.
[0113]Pure human IgG (Alpha Diagnostics Intl. Inc, Cat#20007-1-100), Poly Ethylene Glycol 3350 (PEG, Sigma-Aldrich, Cat#P4338-500G), Water, molecular biology reagent grade (Sigma-Aldrich, Cat#W4502).
[0114]Methods.
[0115]Human IgG and PEG 3350 in powder form were weighed out and mixed into a solution using molecular biology reagent grade water. The percentage of IgG and PEG are 90% and 10% respectively and the powders were dissolved in water at 40 mg / ml concentration. Batches using different IgG mass capacity were prepared: 100 mg (batch 6 and 7), 140 mg (batch 8) and 60 mg of IgG (batch 9). The aqueous solution was placed in a silicone plate and then evaporated in a vacuum chamber with desiccant inside of a refrigerator for a minimum of 19 hours (batch 6, 7 and 8) and up to 21 hours (batch 9) until full evaporation occurs. Data for batches 1-5 are not included because these batches were trial batches made using a different proc...
example 2
Micro-Tablets Comprising Human IgG PEG and Other Excipients
[0119]Materials.
[0120]Pure human IgG (Alpha Diagnostics Intl. Inc, Cat#20007-1-100), Poly Ethylene Glycol 3350 (PEG, Sigma-Aldrich, Cat#P4338-500G), Water, molecular biology reagent grade (Sigma-Aldrich, Cat#W4502), sodium chloride (Sigma-Aldrich, Cat#S9888), mannitol (Sigma-Aldrich, Cat#M8429-100G).
[0121]Methods.
[0122]Human IgG was dissolved along with lubricant PEG 3350 and principal excipients in HUMIRA pen (sodium chloride and mannitol) in the same percentage that in the pen solution. The powders were brought into solution using 0.94 ml of molecular biology reagent grade water. The evaporation process was done using the same procedure as used in a) above.
TABLE 2Micro-tablet Data and IgG recoveries in IgG Micro-tabletFormulation7.5%PEG8.4%3350NaCl67.8% IgGMicro-Micro-16.3% MannitolTotal BalltablettabletMicro-tabletAbsoluteMillingMassLengthWeightDensityMicro-tabletIgG Batch #Ball(grams)(mm)(mg)(mg / mm3)IgG Recovery71 S. Ste...
example 3
Micro-Tablets Comprising HUMIRA and HUMIRA Pen Excipients
[0128]Materials.
[0129]HUMIRA pens (Abbott Laboratories) and Poly Ethylene Glycol 3350 (PEG, Sigma-Aldrich, Cat#P4338-500G).
[0130]Methods.
[0131]The solution contained in the HUMIRA pen was placed in a low-bind 1.5 ml tube where PEG 3350 amount was added and mixed with HUMIRA ingredients. The solution was evaporated following the same conditions as the ones described in example 1 a) and b).
[0132]The milling conditions were the same as in example 1 a) where two balls were used with total mass of 0.5 grams and 1.5 hours (batch 1, 2 and 4) and 1.75 hours (batch 3) of milling duration. The same temperature conditions were kept as in example 1.
[0133]After powder milling, micro-tablets were formed by using a semiautomatic fixture using approx. 3 lbs. of force for compression and a holding compression time of approx. 3 sec. The intact HUMIRA recovered in before-milling powder, after-milling powder and micro-tablets were tested using an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com