Gene-modified non-human animal expressing human gpc3 polypeptide
a technology of gpc3 and non-human animals, which is applied in the field of gene-modified non-human animals expressing a human gpc3 polypeptide, can solve the problems of difficult regulation of expression, difficult substitution in the region, and no generally effective method for producing non-human animals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Human GPC3 Knock-in Mouse
(1) Construction of Knock-in Vector
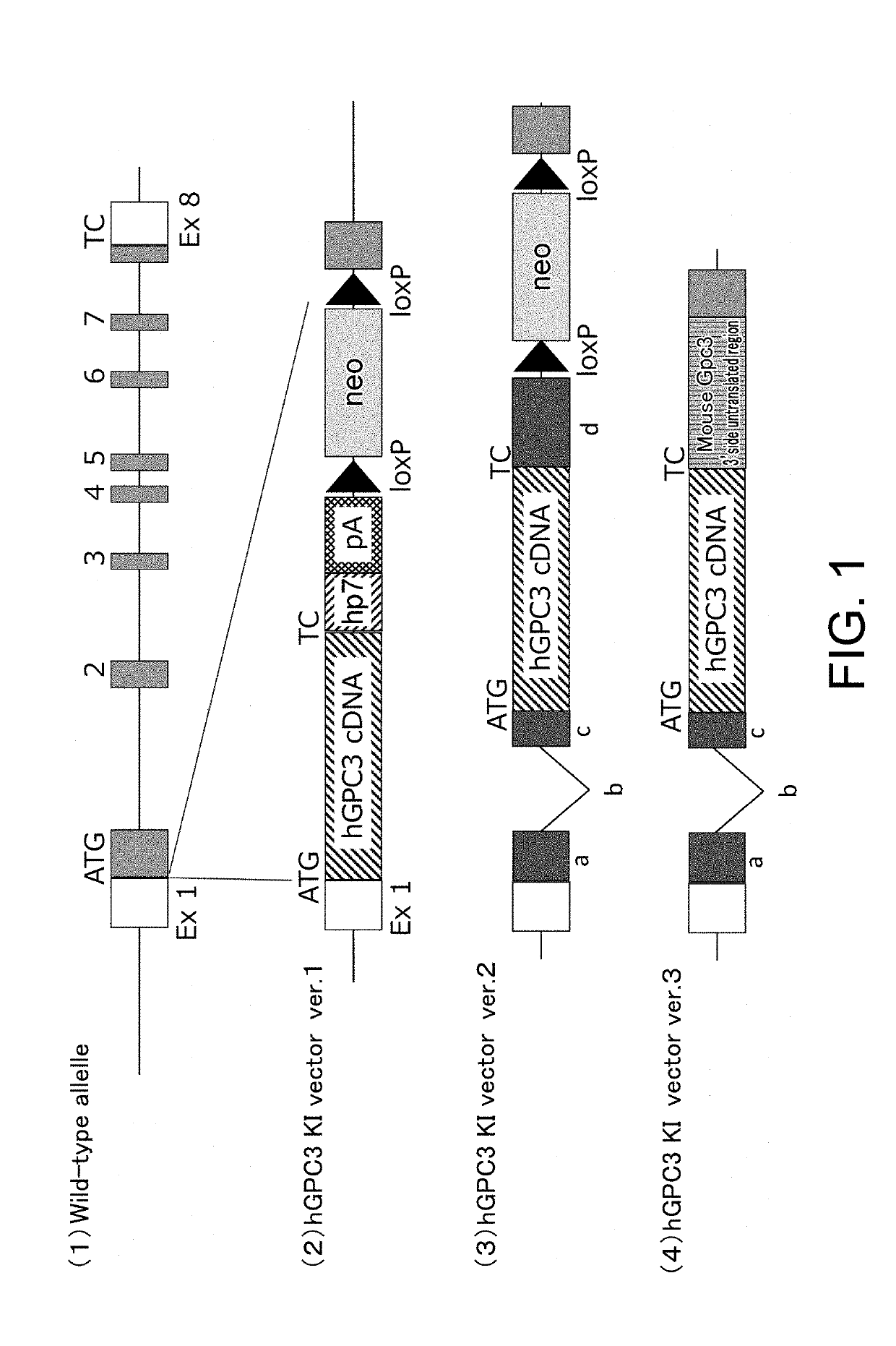
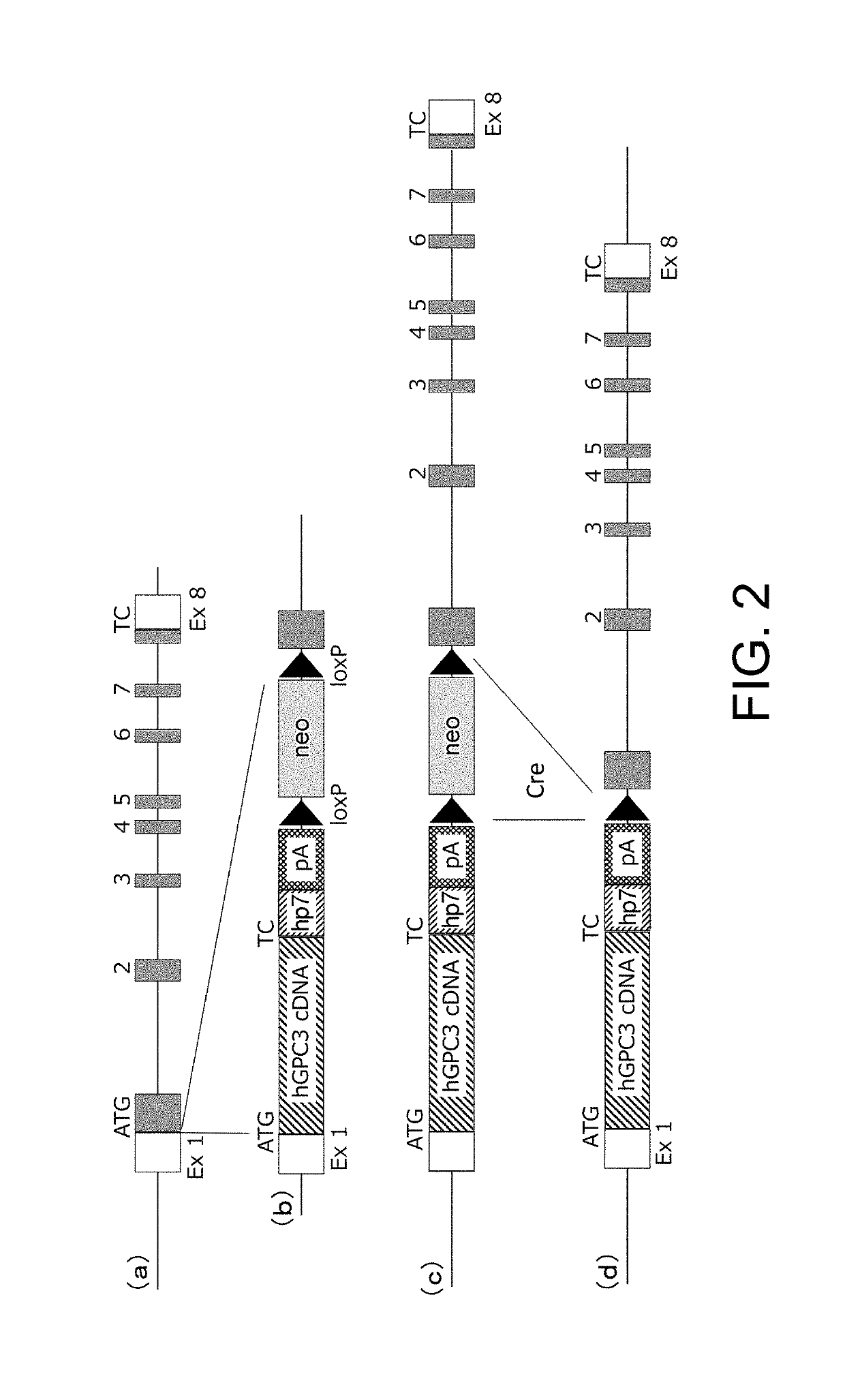
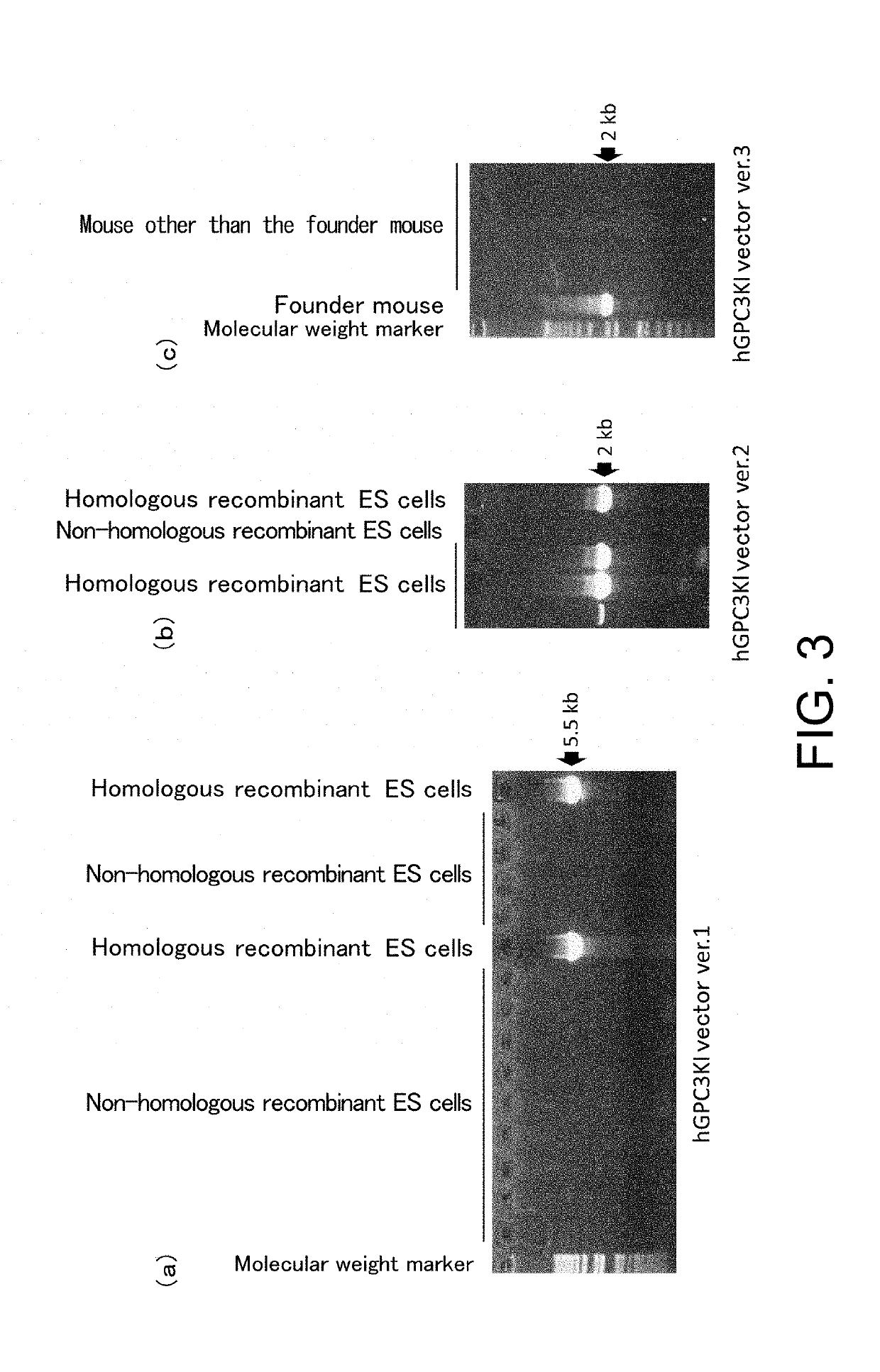
[0287]For hGPC3 knock-in (hGPC3KI) vector ver. 1, an artificial chromosome (bacterial artificial chromosome (BAC)) clone was used, into which the genomic region of the mouse glypican-3 gene (mGpc3) was cloned. The coding sequence (SEQ ID NO: 10) of the human glypican-3 gene (hGpc3), an hp7 sequence (SEQ ID NO: 11), a polyadenylation signal (SEQ ID NO: 12), loxP sequences (SEQ ID NO: 7), and a neomycin resistance (neo) gene (SEQ ID NO: 13) were introduced to the target region of the Gpc3 gene on the BAC clone through homologous recombination using Red / ET System (Gene Bridge). In the introducing, the translation initiation site of hGPC3 was inserted such that it comes to the very position where the translation initiation site of mGpc3 was. The construct of the vector is shown in FIG. 1(2).
[0288]For hGPC3KI vector ver. 2, the approximately 800-bp 5′ upstream region of the mGpc3 gene target region; the mouse beta globin se...
example 2
n Analyses of hGPC3KI Mice
(1) Confirmation of Protein Expression
[0305]Expression of each GPC3 protein was confirmed by Western blotting using lung lysates of hGPC3KI mice and wild-type mice. The lung lysates of hGPC3KI mice and wild-type mice were prepared from one of the two lungs. To prepare samples for SDS-PAGE, a sample buffer (nacalai, catalogue no.) was added to each lung lysate, then heated at 95° C. for five minutes, and diluted to adjust the total amount of protein per lane to 50 μg to 450 μg. After fractionation by SDS-PAGE, proteins were transferred to a membrane. Detection was carried out by using an anti-human GPC3 antibody as the primary antibody, and an HRP-labeled anti-human IgG antibody as the secondary antibody. Tubulin alpha was used as the internal standard of the lysate, and detected using an anti-tubulin alpha antibody as the primary antibody, and an HRP-labeled anti-rat IgG antibody as the secondary antibody.
example 3
lerance to a Human GPC3 Polypeptide
(1) Antigen Immunization
[0315]A soluble human GPC3 protein mixed with an adjuvant (Gerbu adjuvant 10, manufactured by Gerbu GmbH) was administered subcutaneously at 0.1 mg / 0.1 mL to hGPC3KI mice ver. 3 and wild-type mice. The day of first administration was defined as day 0, and the administration was carried out on days 14, 21, 28, 35, 42, 49, and 56. The soluble human GPC3 protein was produced according to a method known to those skilled in the art.
(2) Measurement of Antibody Titer
[0316]The day of first administration was defined as day 0, and blood was collected with heparin treatment before each administration to obtain plasma. Concentration of the anti-human GPC3 antibody in plasma was measured by enzyme linked immunosorbent assay (ELISA) where a soluble human GPC3 (shGPC3) was coated. Coating was carried out using 4 μg / mL shGPC3 solution (pH9.6) on a 96-well plate. Each of the wells was washed with a PBS solution containing 0.05% tween20, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com