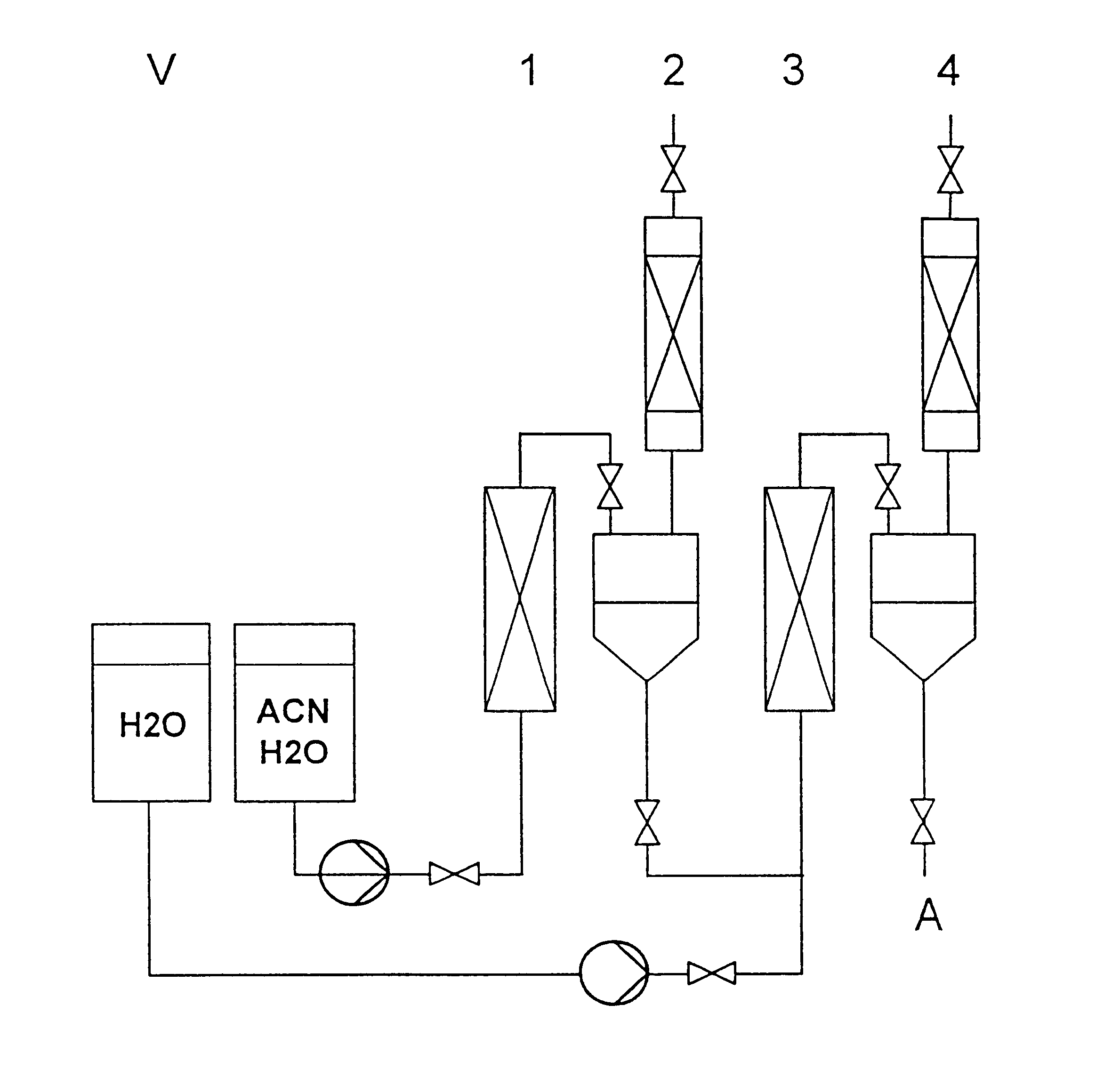
Continuous preparation of polyamides from aminonitriles
a technology of aminonitrile and polyamide, which is applied in the field of continuous preparation of polyamides from aminonitriles, can solve the problems of affecting the construction of high molecular weight polymers, affecting the production of polyamides, and virtually impossible to remove catalysts in either process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Improvement in relative product viscosity coupled with simultaneous increase in throughput by 50% through use of catalyst in process stage 1.
Purity of aminocapronitrile used: 99%.
example 3
Dependence of relative product viscosity on purity of aminocapronitrile used and on process parameters. Catalyst use in process stage 1.
example 4
Improved product properties, i.e., increased molecular weight and increased carboxyl end group (CEG) content of end product, through use of catalyst in process stages 1 and 2.
Purity of aminocapronitrile used: 99%
Molar ACN:water mixing ratio=1:6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com