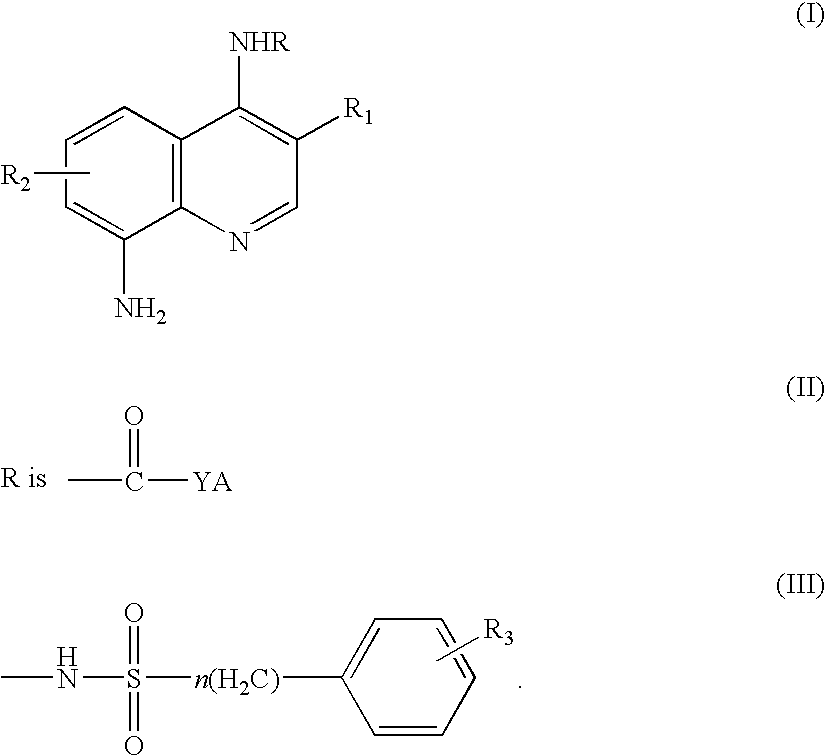
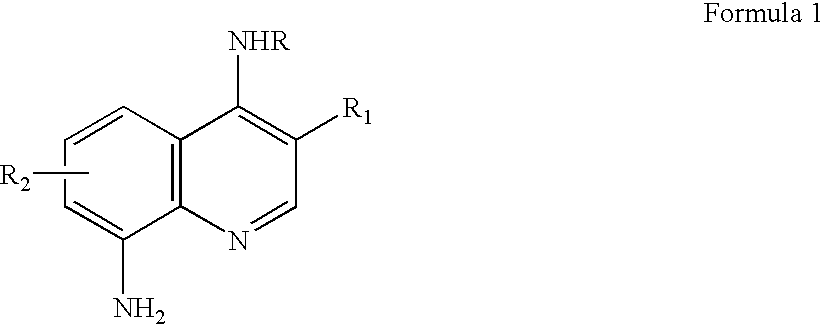
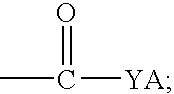
Quinoline derivatives as caspase-3 inhibitor, preparation for producing the same and pharmaceutical composition comprising the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
Example 1
Synthesis of 2-[(2-Nitro-phenylamino)-methylene]-malonic acid diethyl ester (Compound of Formula 4)
[0152]To a solution of 2-nitroaniline (20 g, 144.8 mmol) dissolved in 400 ml of dry-ethanol in a 500 ml round bottom flask was added dimethylethoxymethylene malonate (34 g, 159.3 mmol). The solution was heated and refluxed for 6 hrs at 120° C. with stirring. The reaction was monitered by TLC (n-hexane / ethyl acetate=3 / 1). After completing the reaction and cooling to room temperature, the resulting precipate was collected by filtration, and the residue was washed three times with 100 ml of n-hexane to give 2-[(2-Nitro-phenylamino)-methylene]-malonic acid diethyl ester (31 g, 100.6 mmol, yield 69%).
[0153]1H NMR (300 MHz, CDCl3): δ=8.55 (d, 1H, —NHCHC(CO2CH2CH3)2, J=13.00 Hz), 8.28 (dd, 1H, aromatic H, J=1.25, 1.19 Hz), 7.68 (t, 1H, aromatic H, J=4.06 Hz), 7.52 (d, 1H, aromatic H, J=8.34 Hz), 7.23 (m, 1H, aromatic H), 4.42 (q, 2H, —NHCHC(CO2CH2CH3)2), 4.30 (q, 2H, —NHCHC(CO2CH2CH3...
example 2
Synthesis of 8-Nitro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid ethyl ester (Compound of Formula 5)
[0154]A solution of 2-[(2-Nitro-phenylamino)-methylene]-malonic acid diethyl ester (10 g, 32.44 mmol) dissolved in 20 ml of diphenyl ether in a 250 ml round bottom flask was heated and refluxed for 5 hrs at 280° C. with stirring. The reaction was monitered by TLC (n-hexane / ethyl acetate=3 / 1). After completing the reaction and cooling to room temperature, 200 ml of diethylether was added to the mixture and the resulting precipitate was collected by filtration, and the residue was washed with 200 ml of diethyl ether to give 8-Nitro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid ethyl ester (6.5 g, 19.08 mmol, yield 59%).
[0155]1H NMR (300 MHz, CDCl3): δ=11.40 (s, 1H, —NHCH—), 8.88 (d, 1H, —NHCH—, J=7.17 Hz), 8.69 (d, 2H, aromatic H, J=6.84 Hz), 7.56 (t, 1H, aromatic H, J=4.06 Hz), 4.42 (q, 2H, —CH2CH3), 3.39 (t, 3H, —CH2CH3, J=7.08 Hz)
example 3
Synthesis of 8-nitro-4-chloro-quinoline-3-carboxylic acid ethyl ester (Compound of Formula 6)
[0156]To a solution of 8-Nitro-4-oxo-1,4-dihydro-quinoline-3-carboxylic acid ethyl ester (10 g, 38.14 mmol) in 100 ml of thionyl chloride in a 250 ml round bottom flask was added a catalytic amount of 200 ul of dimethyl acetamide. The mixture was heated and refluxed for 2 hrs with stirring. The reaction was monitered by TLC (n-hexanelethyl acetate=3 / 1). After completing and cooling to room temperature, thiony chloride was removed under vacuum and 100 ml of water was added. The residue was extracted three times with 100 ml of ethyl acetate each time and the organic layer was dried over magnesium sulfate and filtrated. The residue was purified by column chromatography (hexan / ethyl acetate=3 / 1) to give 8-nitro-4-chloro-quinoline-3-carboxylic acid ethyl ester (9.5 g, 33.85 mmol, 89%)
[0157]1H NMR (300 MHz, CDCl3) δ=9.35 (s, 1H, —N═CH—), 8.67 (d, 1H, aromatic H, J=9.38 Hz), 8.17 (t, 1H, aromatic H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com