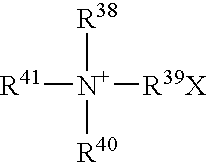Water-stabilized antimicrobial organosilane products, compositions, and methods for using the same
a technology of antimicrobial organosilane and water-stabilized compositions, which is applied in the direction of biocide, detergent compounding agents, liquid soaps, etc., can solve the problems of products notorious for agitation difficulty, unstable water, and organicsilanes in water, so as to improve the ease of dissolution in water, improve the stability of water composition, and facilitate the effect of diluted water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Non-Acidic Stabilizing Solutions
[0377]Five different antimicrobial organosilane-based compositions were prepared and exposed to different storage temperatures. Composition A is a 10% active composition containing 10 wt % 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride, 2 wt % N-alkyl,N,N dimethyl N benzylammonium chloride (CAS #68424-85-1), 2 wt % diethylene glycol butyl ether, and water. The composition was not acidic. A portion of composition A was stored in a refrigerator cooled to 2° C. for approximately 27½ hours after which the color of composition A was white, similar to milk. The white color indicated that the 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride self-condensed. A second portion of composition A was stored at 60° C. for approximately 28 hours after which the composition remained clear in color. The clear color indicated that the 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride did not self-condense.
[0378]Composition B is a 15%...
example 2
Varying the Type of Surfactant in the Stabilizing Solution
[0385]Six different antimicrobial organosilane compositions were prepared with different combinations of surfactants in the stabilizing solution. Composition C, a 15% active composition, was prepared from 20.83 grams of 3-(trim ethoxysilyl)propyldimethyloctadecyl ammonium chloride mixed with a stabilizing solution consisting of 1.5 grams of VIDET Qx9 (Janesville, Wis.), 1.5 grams of N-alkyl,N,N dimethyl N benzylammonium chloride (CAS #68424-85-1), and 2.0 grams diethylene glycol butyl ether. VIDET Qx9 is a cationic surfactant blend obtained from Vitech International Inc. A first sample of composition C was stored in a refrigerator cooled to 2° C., and a second sample was stored at 60° C. The cold storage sample was milky white in color after about 24 hours. The heated storage sample was thin and clear after about 28 hours. Again, the milky white color of the frozen sample indicated that the 3-(trimethoxysilyl)propyldimethyloc...
example 3
[0391]A stabilizing solution was prepared from 2 grams of N-alkyl,N,N dimethyl N benzylammonium chloride (CAS #68424-85-1), 57.17 grams of H20, and 20 grams of diethylene glycol butyl ether The pH was adjusted to 2 with an acid. The stabilizing solution was well mixed with light heat with 20.83 grams of 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride. After 20 days, the composition I was clear and thin indicating that 3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride did not polymerize and was stable in the presence of water.
[0392]A second stabilizing solution was prepared from 2 grams of N-alkyl,N,N dimethyl N benzylammonium chloride (CAS #68424-85-1), 57.17 grams of H20, 20 grams of VIDET Q-3. The pH was adjusted to 2 with the addition of an acid. The stabilizing solution was well mixed with light heat with 20.83 grams of (3-(trimethoxysilyl)propyldimethyloctadecyl ammonium chloride). After 20 days, the composition J was clear and slightly viscous.
[0393]Com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com