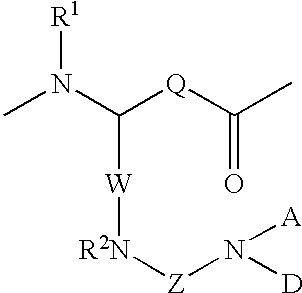
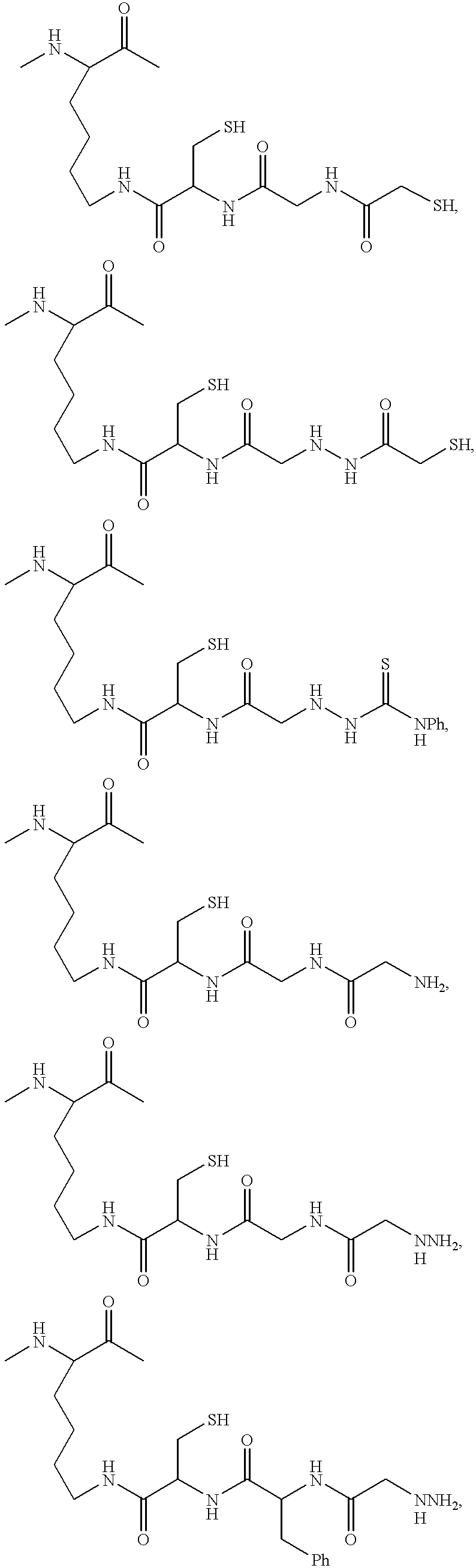
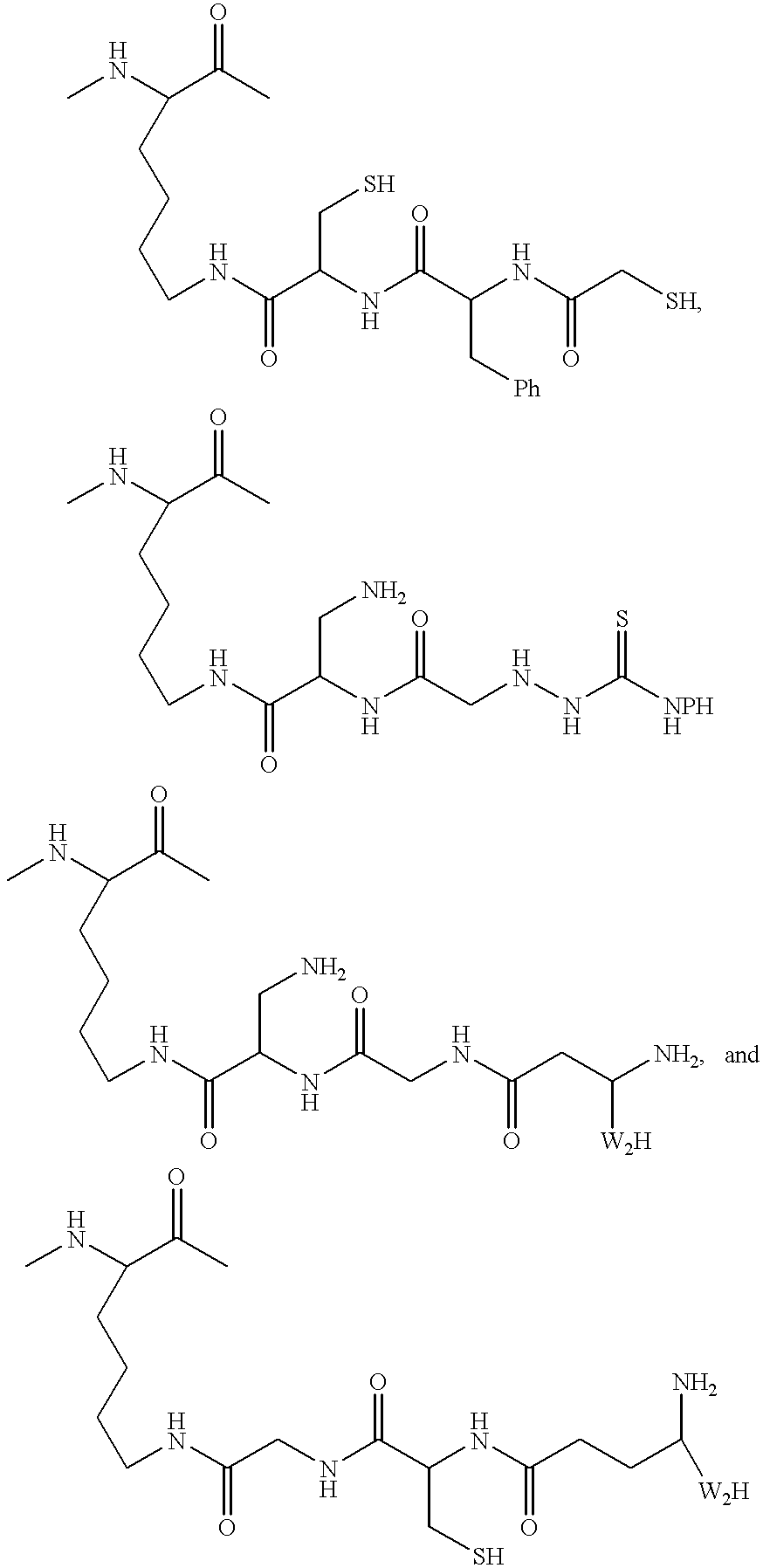
Radiometal-binding analogues of luteinizing hormone releasing hormone
a technology of luteinizing hormone and radiometal, which is applied in the field of derivatives of leutenizing hormone releasing hormone, can solve the problems of limiting the radiometal which can be used, altering the receptor binding properties of the compound, and being unstable and unstabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of N.sup..alpha. Alloc-Ne-Fmoc-L-Lycine
N.sup.e -Fmoc-L-Lysine (10.00 g, 27.1 mmol, 100 mol %, Bachem Biosciences, Inc.) was suspended in dioxane (100 ml) and Na.sub.2 CO.sub.3 (1M, 33 ml) to form a milky suspension. Allyl chloroformate (3.2 ml, 30.2 mmol, 111 mol %) was added to dioxane (10 ml) and this solution was added dropwise to the suspension of N.sup.e -Fmoc-L-Lysine over 10 min. Sodium carbonate, (1M, 20 ml) was added in two portions and an additional quantity of allyl chloroformate (0.3 ml) was added. The reaction was stirred at room temperature for 16 hours. The volatile solvents were removed under reduced pressure and the residue was washed with diethyl ether (50 ml). The residual liquid was then acidified with HCl (1M) and extracted with ethyl acetate (2.times.150 ml). The organic layers were combined, washed with saturated NaCl (50 ml), dried over Na.sub.2 SO.sub.4, evaporated under reduced pressure to obtain a crude oily product (16 g). The crude product was ...
example 2
Synthesis of 2-(triphenylmethylmercapto)acetyl hydrazide
2-(triphenylmethylmercapto) acetic acid (20.35 g, 60.9 mmol, 100 mol %) was dissolved in anhydrous THF (150 ml) and cooled in an ice water bath. t-Butylcarbazate (8.61 g, 65.1 mmol, 107 mol %) was added to the reaction solution followed by diisopropylcarbodiimide (10.0 ml, 63.9 mmol, 105 mol %). The reaction was allowed to warm slowly to room temperature and stirred for 28 hours. The reaction mixture was filtered to remove the white precipitate that had formed and the filtrate was concentrated to a white foam by removal of the solvent under reduced pressure. This material was dissolved in chloroform (75 ml). Then acetic acid (75 ml) was added followed by the addition of borontrifluoride etherate (10.0 ml, 81 mmol, 134 mol %). The reaction was stirred at room temperature for 6 hours and then quenched by pouring the reaction mixture into water (200 ml) containing sodium acetate (30 g). This mixture was extracted with chloroform (...
example 3
Synthesis of N.sup..beta. -[2-(triphenylmethylthio) acetyl]azaglycine
Glyoxylic acid monohydrate (0.59 g, 6.41 mmol, 110 mol %) was dissolved in methanol (20 ml) and 2-(triphenylmethylmercapto)acetyl hydrazide (2.03 g, 5.82 mmol, 100 mol %) was added. Dioxane (20 ml) was added to the cloudy reaction mixture and the reaction was stirred at room temperature for 18 hours. Sodium borohydride (1.76 g) was added to the reaction mixture and after 30 minutes, another quantity of sodium borohydride (0.60 g) was added. The reaction was stirred for 3 hours at room temperature, then quenched by pouring the reaction mixture into HCl (1M, 60 ml). The mixture was extracted with ethyl acetate (2.times.50 ml). The organic layers were combined, washed with saturated NaCl solution (40 ml), dried over Na.sub.2 SO.sub.4, filtered, and concentrated under reduced pressure on the rotary evaporator to afford a solid (2.5 g) having ESMS MH.sup.+ calculated 407, found 407.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Power | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
| Power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com