Glyphosate formulations containing etheramine surfactants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
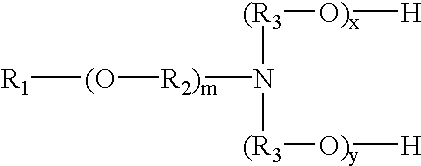
The surfactant used in Example 1 is a tertiary etheramine having the chemical structure represented above in which R.sub.1 is C.sub.12 -C.sub.14 alkyl, m is 3, x+y is 5 and R.sub.2 and R.sub.3 are each ethylene.
The aqueous concentrate composition of Example 1 was prepared by mixing the following ingredients in the order given: (1) aqueous solution of glyphosate isopropylamine salt containing 46% glyphosate a.e., 67.4 g. (2) surfactant as defined above, 10.0 g. (3) deionized water, 22.6 g.
The composition can be calculated to contain 31% glyphosate a.e. and 10% surfactant. Specific gravity of the composition at 20 / 15.6.degree. C. was determined to be 1.1628. Cloud point of the composition was >90.degree. C.
example 2
The surfactant used in Example 2 is the same as that used in Example 1. The aqueous concentrate composition of Example 2 was prepared by mixing the following ingredients in the order given: (1) aqueous solution of glyphosate isopropylamine salt containing 46% glyphosate a.e., 1348 g. (2) surfactant as defined above, 110 g. (3) deionized water, 542 g.
The composition can be calculated to contain 31% glyphosate a.e. and 5.5% surfactant. Specific gravity of the composition at 20 / 15.6.degree. C. was determined to be 1.1630. Cloud point of the composition was >90.degree. C.
The composition of Example 2 was submitted for eye irritation testing according to the standard procedure prescribed in US Environmental Protection Agency (EPA) Publication 540 / 9-82-025, November 1982, entitled Pesticidal Assessment Guidelines, Subdivision F. Hazard Evaluation: Human and Domestic Animals. The study was conducted in compliance with EPA Good Laboratory Practice (GLP) standards. Results were obtained placi...
example 3
The surfactant used in Example 3 is a tertiary etheramine having the chemical structure represented above in which R.sub.1 is C.sub.12 -C.sub.14 alkyl, m is 2, x+y is 5, R.sub.2 is isopropylene and R.sub.3 is ethylene.
The aqueous concentrate composition of Example 3 was prepared by mixing the following ingredients in the order given: (1) aqueous solution of glyphosate isopropylamine salt containing 46% glyphosate a.e., 1348 g. (2) surfactant as defined above, 200 g. (3) deionized water, 452 g.
The composition can be calculated to contain 31% glyphosate a.e. and 10% surfactant. Specific gravity of the composition at 20 / 15.6.degree. C. was determined to be 1.1618. Cloud point of the composition was >90.degree. C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com