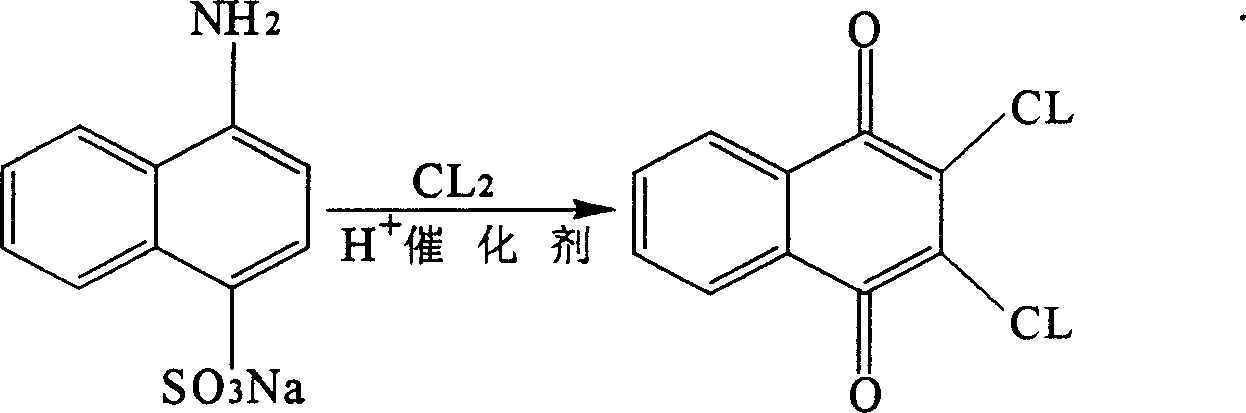
Preparation method of 2,3-dichlor-1,4-naphthaquinones
A technology of naphthoquinone and naphthylamine, applied in 2 fields, to achieve the effect of heavy recovery workload, large solvent loss and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
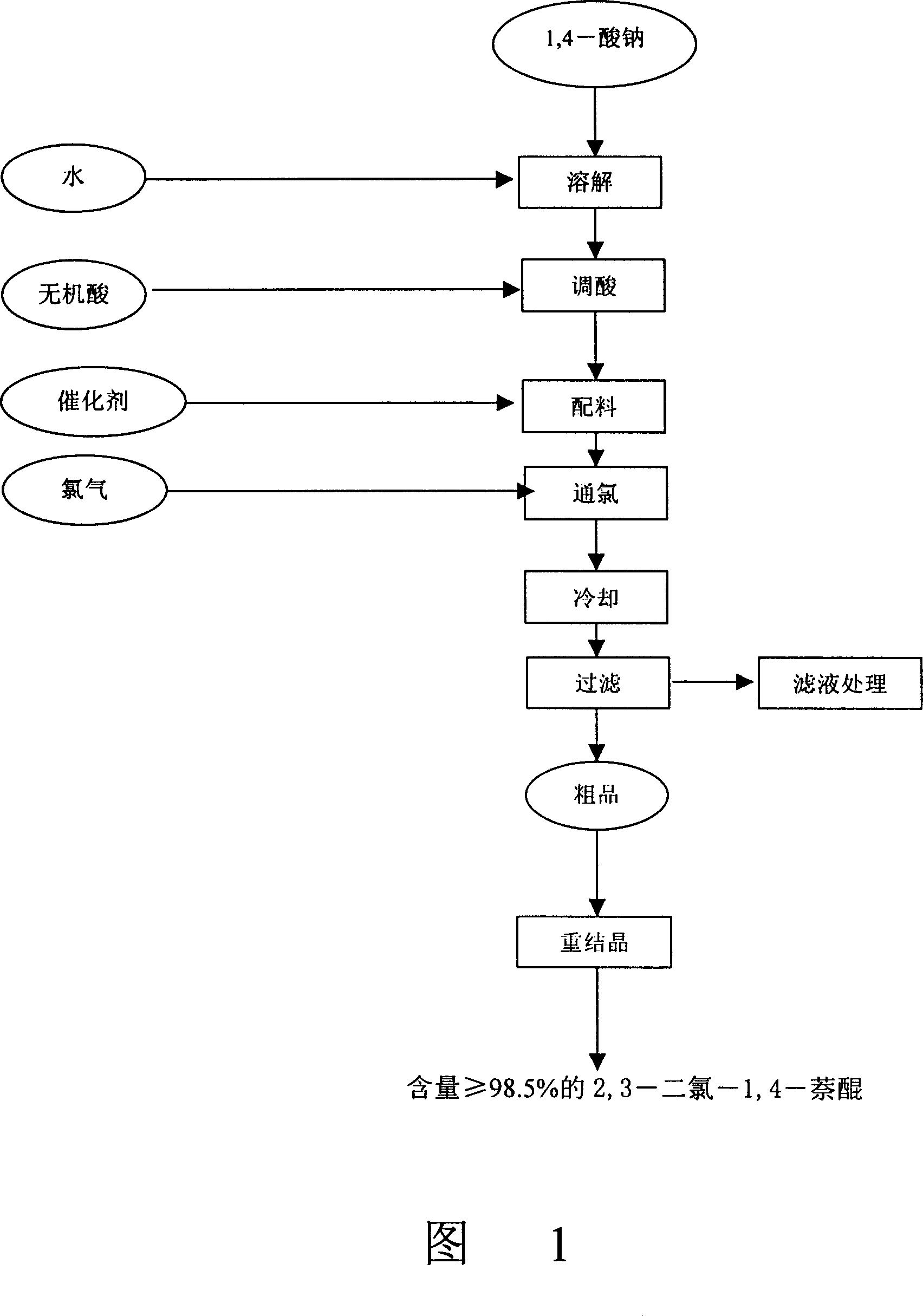
[0034] (1) Sodium 1,4-acid dissolved
[0035] Add 150Kg of sodium 1,4-acid into the reaction pot, add 500Kg of water, heat up to stir and dissolve. Add hydrochloric acid dropwise to control the pH value from 2 to 5, and stir for 10 minutes.
[0036] (2) Chlorine
[0037] Add 5Kg of iron powder and stir for 15 minutes. Feed chlorine gas, control the temperature at 30-90° C., react for 20 hours, until the reaction is complete (the analysis shows that no raw material 1,4-acid sodium exists), and then stop the chlorine flow. Cool to 35°C and filter to dryness.
[0038] (3) Recrystallization
[0039] To obtain 150Kg of crude product (content: 95%), add 750Kg of ethanol and heat up to reflux. Cool to 30°C. Suction filter to dryness. Control the temperature of the oven at 50-70° C., bake for 5 hours until dry, and obtain 110 Kg of the product (2,3-dichloro-1,4-naphthoquinone) with a content of more than 98.5%.
Embodiment 2
[0041] Change hydrochloric acid into sulfuric acid in embodiment 1, obtain product (2,3-dichloro-1,4-naphthoquinone) 108Kg, content more than 98.5%
Embodiment 3
[0043] Change hydrochloric acid into phosphoric acid in embodiment 1, obtain product (2,3-dichloro-1,4-naphthoquinone) 100Kg, content more than 98.5%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com