Drug- or gene-carrier composition having lowered hemagglutinin activity
A composition and carrier technology, applied in the field of drug carrier composition or gene carrier composition with reduced blood coagulation activity, can solve the problem of drug delivery system with reduced blood coagulation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] [Example 1] Preparation and purification of SeV vector containing NLS-LacZ gene
[0127] The NLS-LacZ / SeV carrying the LacZ gene containing a nuclear localization signal was obtained by a previously published method (Kato et al., Genes Cells, 1996, 1, 569-579; Hasan et al., J.Gen.Virol., 1997, 78 , 2813-2820) prepared. The vector was inoculated into eggs containing 10-day-old embryos. After three days of incubation at 35.3°C, the allantoic fluid was harvested and centrifuged at 4,000 rpm for 15 minutes. The resulting supernatant was then centrifuged at 10,000 rpm for one hour to pellet the vector. After resuspension in PBS, the vector was layered on a sucrose density gradient (30% / 50%) and centrifuged at 25,000 rpm for one hour (in a Beckmanrotor SW28). The vector at the sucrose interface was recovered, centrifuged, pelleted, and then resuspended in PBS to prepare a stock solution of the purified vector (hereinafter described as SeV vector). The protein concentratio...
Embodiment 2
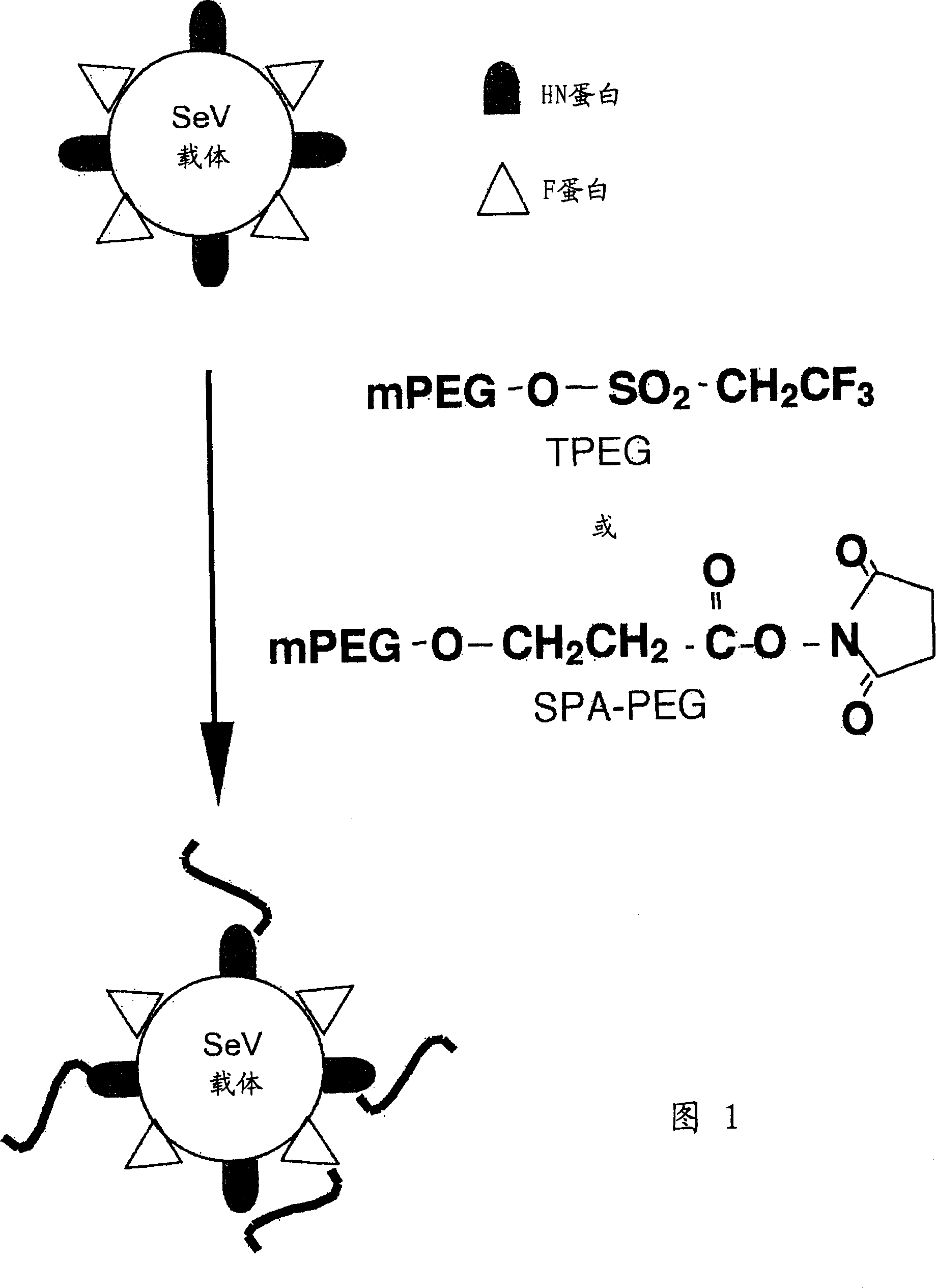
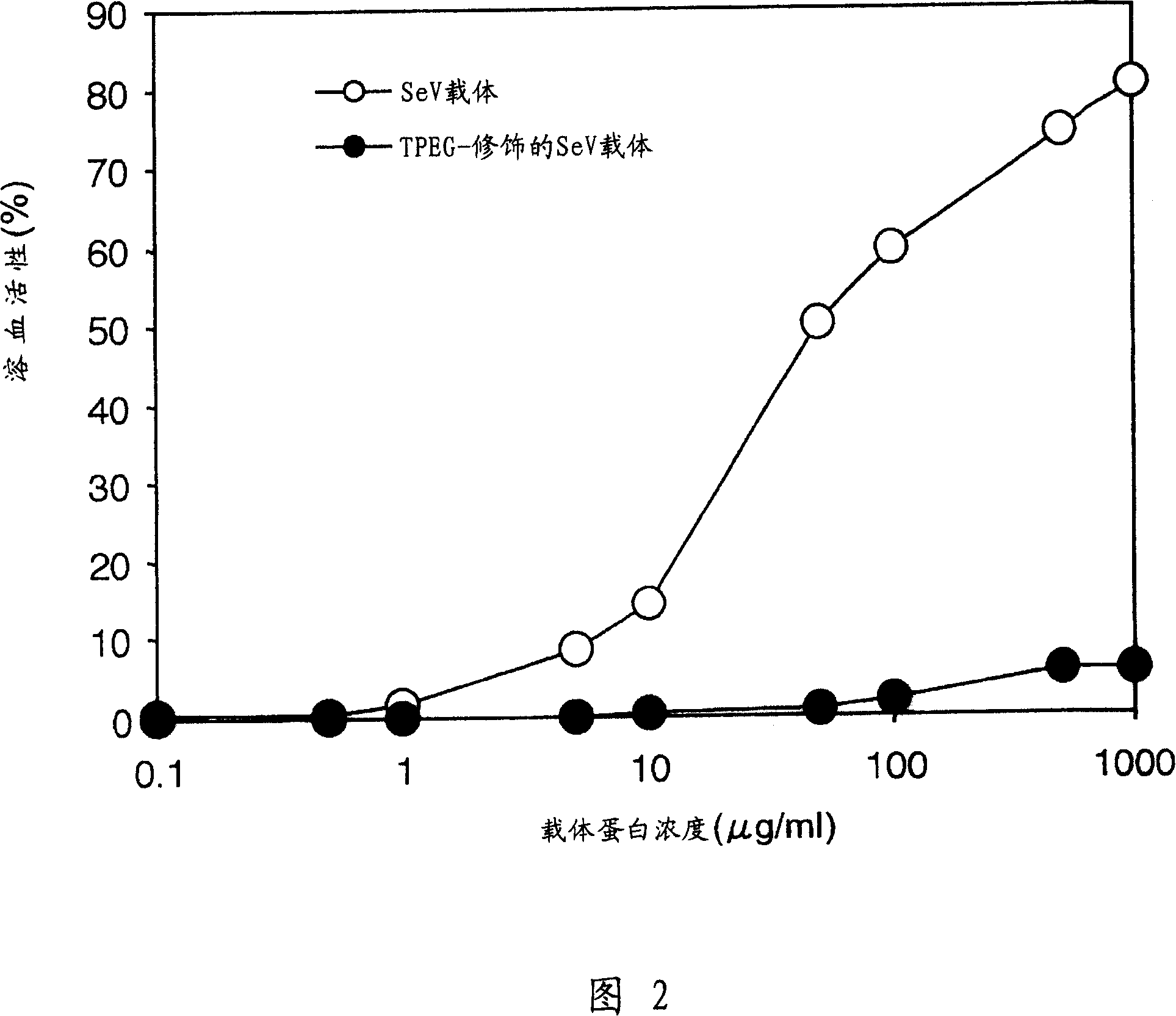
[0128] [Example 2] Preparation of TPEG-modified SeV vector
[0129] The SeV vector stock solution in Example 1 was diluted to 2 mg protein / ml in PBS. To 0.5 ml of this solution was added 0.5 ml of 0.5 M boric acid buffer (pH 8.5) to prepare a solution of 1 mg protein / ml at pH 8.5. 1 mg of Tresyl-activated PEG reagent (TPEG, MW: 5000) (Shearwater Polymers) was added in a small amount to the above solution while stirring, and reacted at room temperature for 90 minutes ( FIG. 1 ). After the reaction was completed, the reaction mixture was diluted with ice-cold PBS (14 ml), and then the carrier was recovered by centrifugation at 15,000 rpm for one hour (in a Beckman rotor SW28.1). The vector was resuspended in PBS (0.8 ml), and a TPEG-modified SeV vector was obtained. The protein concentration and HAU of the modified carrier were determined as in Example 1.
Embodiment 3
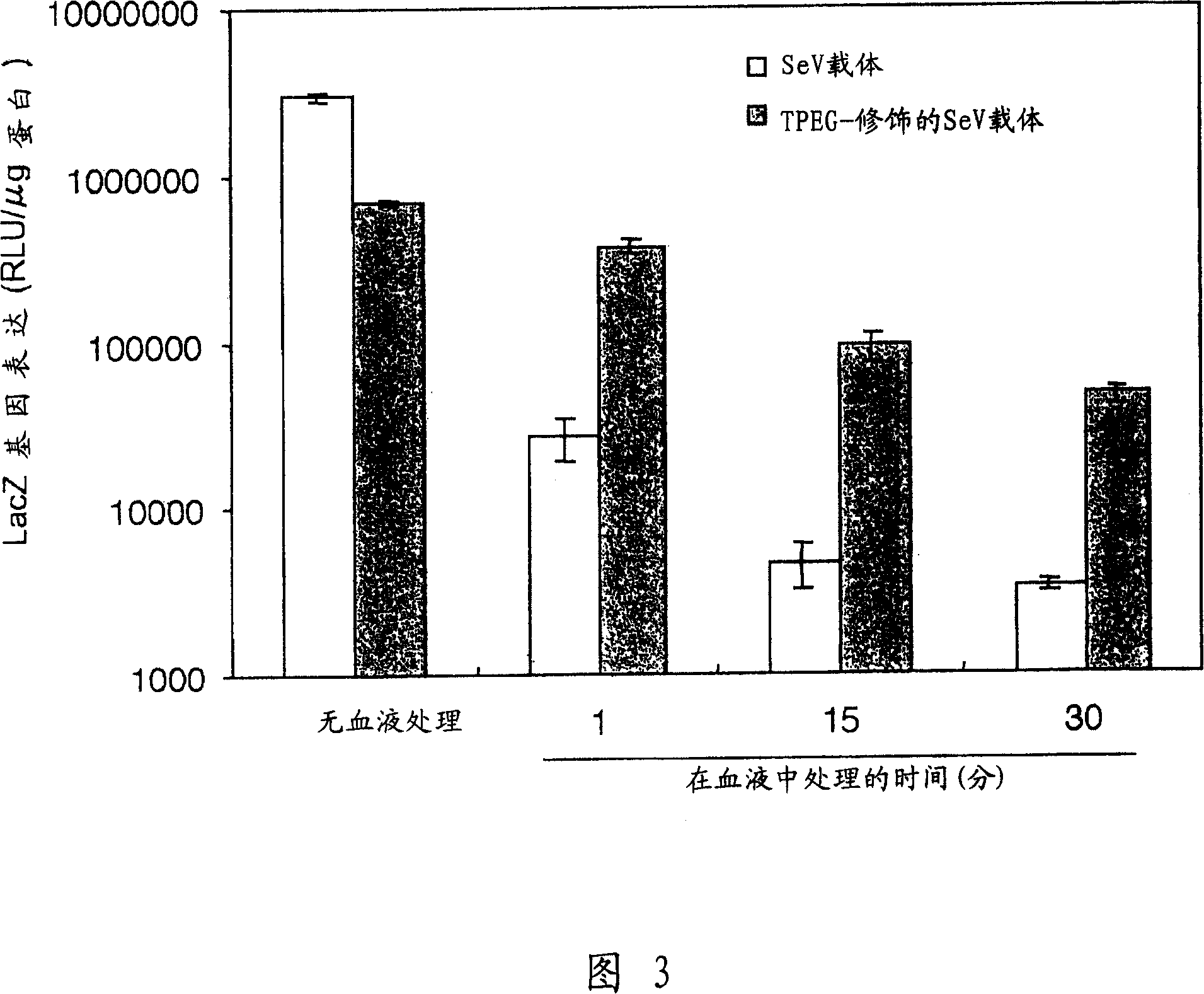
[0130] [Example 3] Utilize TPEG-modified SeV vector to carry out gene expression in vitro
[0131] The SeV vector stock solution in Example 1 was diluted to 1×10 in PBS 5pfu / ml. The protein concentrations of the TPEG-modified SeV vector solution and the non-modified SeV vector solution in Example 2 were standardized, so that the two vectors provided equal numbers of virus particles. The day before the experiment, HeLa cells (from human cervical cancer) were mixed with 5×10 4 The cells / well were cultured (1 ml / well of MEM medium containing 10% inactivated fetal calf serum (FCS) was added) on a 12-well plate (Sumitomo Bakelite). After reducing the medium to 0.5ml, add 50μl of the above-mentioned diluted carrier solution (equivalent to 5×10 3 pfu / well, moi=0.1), in 5% CO 2 Infections were performed at 37°C when present. After one hour, the carrier was removed and the cells were washed twice with medium. Add fresh medium (2ml) to the cells, then in 5% CO 2 Incubate further ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com