Diphenyl ketone photo initiator containing sulfur and its preparing method
A technology of photoinitiator and benzophenone, applied in the field of organic compounds and their preparation, can solve problems such as the decrease of photosensitivity, and achieve the effects of improving photosensitivity, enhancing initiating performance and improving initiating performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a three-necked flask equipped with a water separator, a condenser, and a nitrogen protection device, 11.00 grams of thiophenol, 23.16 grams of 4-amino-4'-chlorobenzophenone, and 40 mL of 1-methyl -2-Pyrrolidone, 6.72 grams of potassium hydroxide and 30 mL of toluene, heated up to 130-135 ° C, reacted for 3 hours, during this period, water azeotroped out with toluene; After 5 hours, cool down to room temperature naturally, slowly pour the filtrate into a mixture of 100mL concentrated hydrochloric acid and 300mL ice-water, after stirring and suction filtration, add the filter cake to concentrated ammonia water, stir for half an hour, and then suction filtration, After washing with water, drying and recrystallization, 20.9 grams of 4-amino-4'-phenylthiobenzophenone was obtained, with a yield of 68.4%; mass spectrum (70eV) m / e: 305; 1 H NMR ([-d6]CDCl 3 , 400MHz): δ=7.69-7.67 (2H, Ar), 7.64-7.62 (2H, Ar), 7.50-7.48 (2H, Ar), 7.41-7.36 (3H, Ar), 7.27-7.24 (2H, Ar) , 6.6...
Embodiment 2
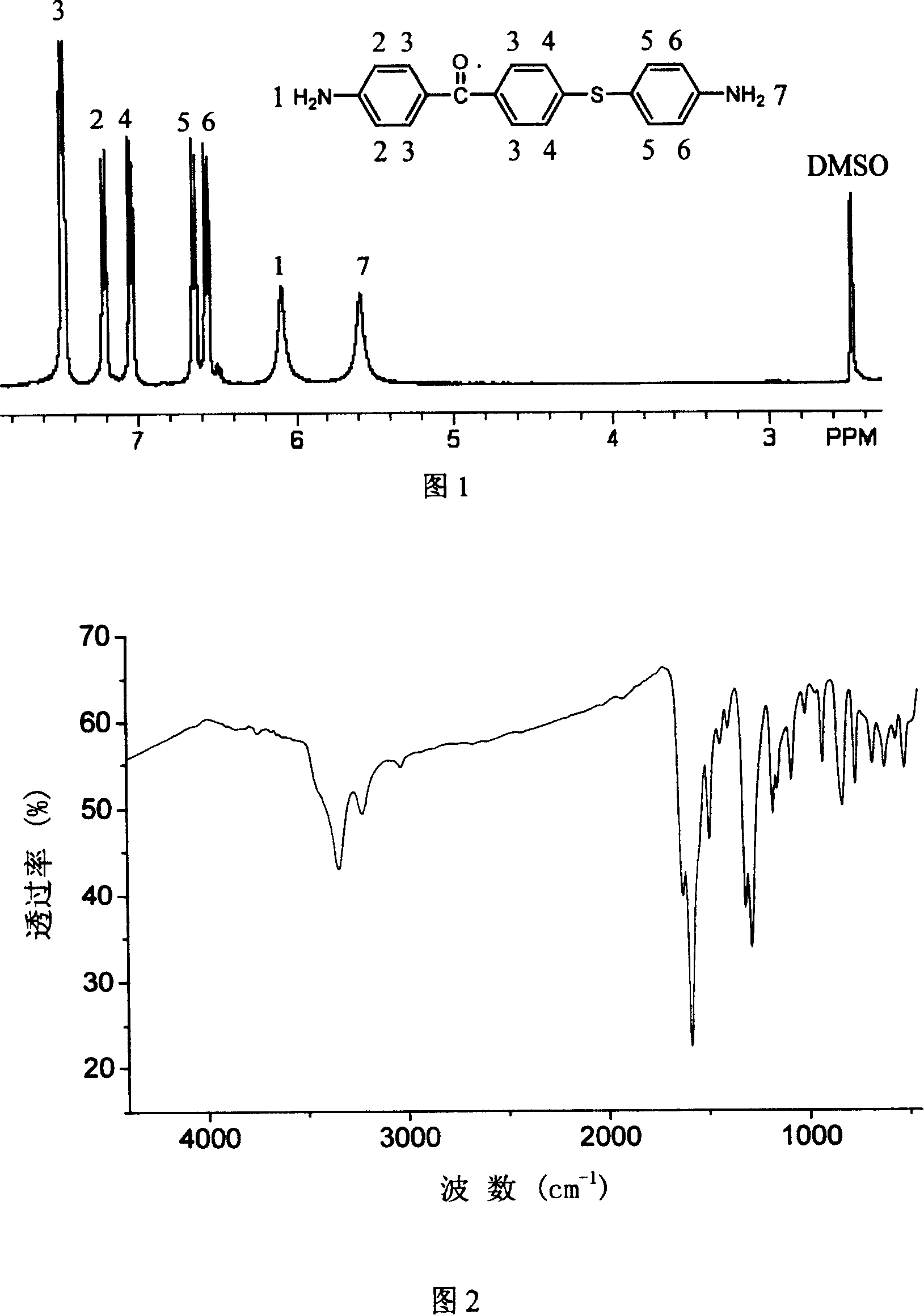
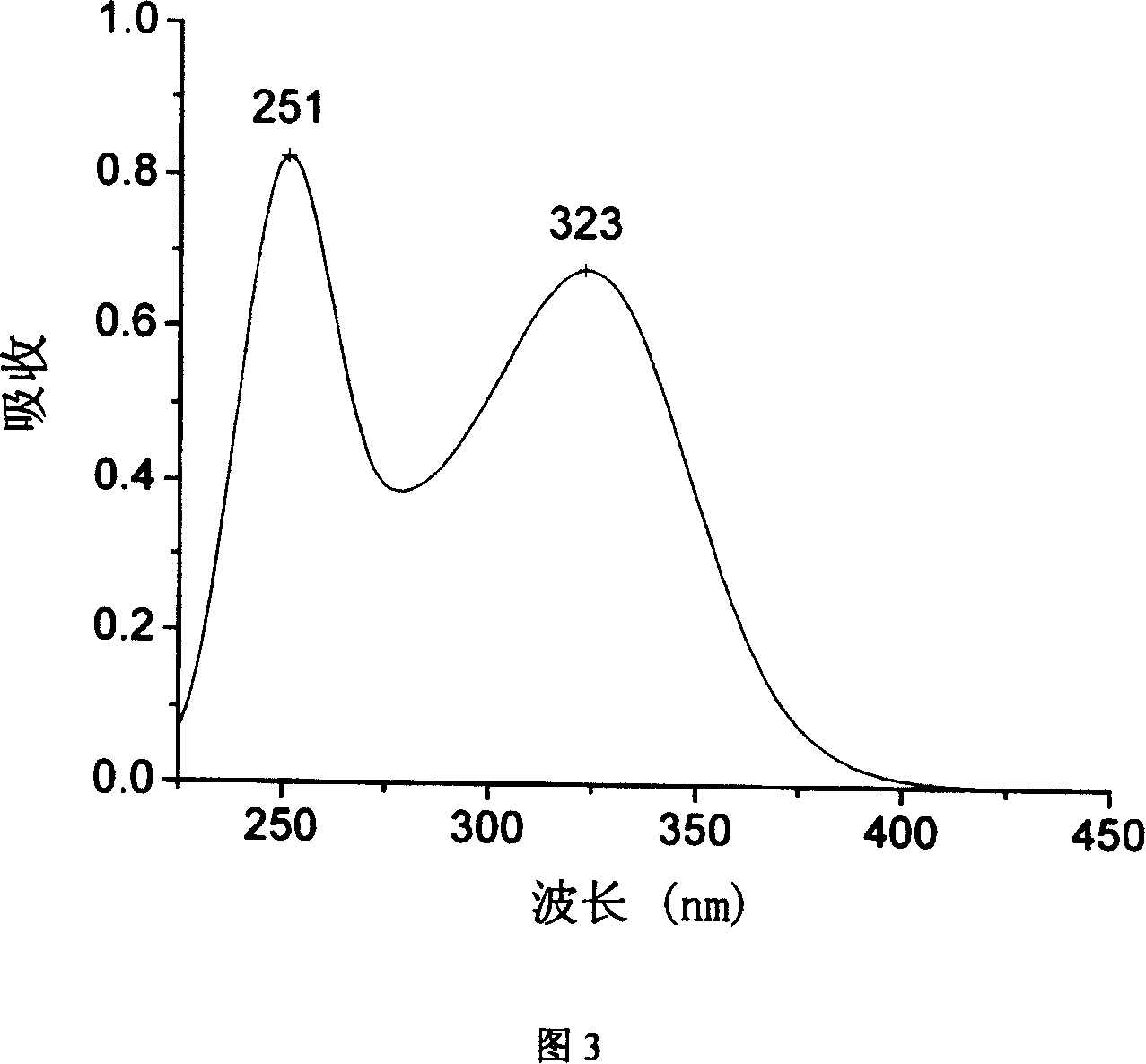
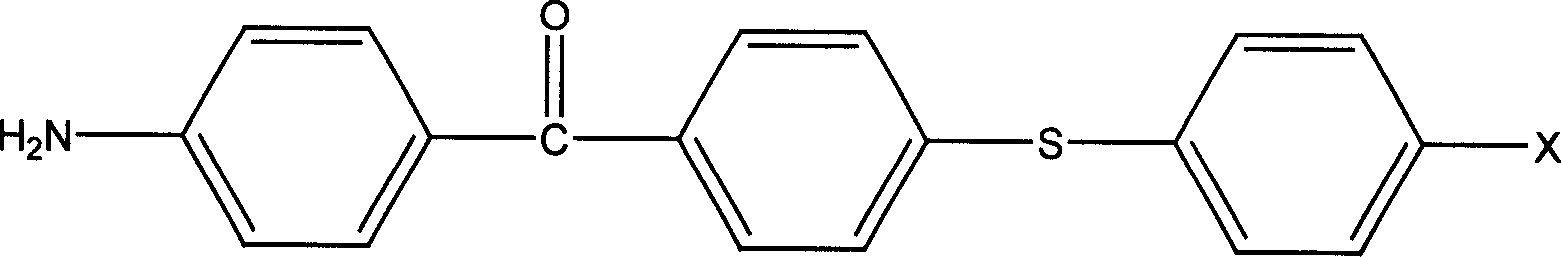
[0023] In a three-necked flask equipped with a water separator, a condenser, and a nitrogen protection device, 12.52 grams of p-aminothiophenol, 23.16 grams of 4-amino-4'-chlorobenzophenone, and 40 mL of N were added sequentially under nitrogen protection. N-Dimethyl-formamide, 6.72 grams of potassium hydroxide and 20 mL of toluene, heated up to 125-130 ° C, reacted for 3 hours, during this period, water azeotroped out with toluene, and then heated up to 160 ° C, all the toluene Separated, reacted for 5 hours, cooled down to room temperature naturally, slowly poured the filtrate into a mixture of 100mL concentrated hydrochloric acid and 500mL ice-water, stirred and filtered with suction, added the filter cake to concentrated ammonia water, stirred for half an hour, and then After suction filtration, water washing, drying and recrystallization, 21.5 grams of 4-amino-4'[4-aminophenylthio]benzophenone were obtained, with a yield of 67.2%; mass spectrum (70eV) m / e: 320; Figure 1 I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com