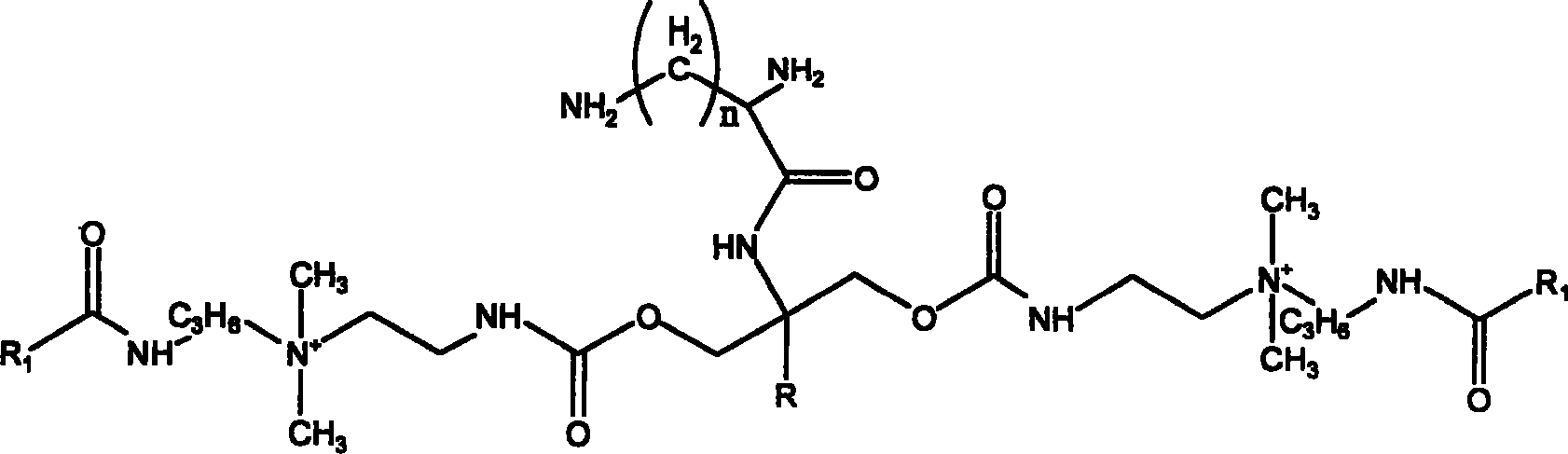
Quaternary bis-ammonium salt diamine fluoride and preparation method thereof
A double quaternary ammonium salt, fluorination technology, applied in chemical instruments and methods, botanical equipment and methods, preparation of organic compounds, etc., can solve the problem of poor Gram-negative bacteria effect, increased material water absorption, monoquaternary The problem of large amount of salt, etc., to achieve the effect of easy source of raw materials, good bactericidal effect, and easy control of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
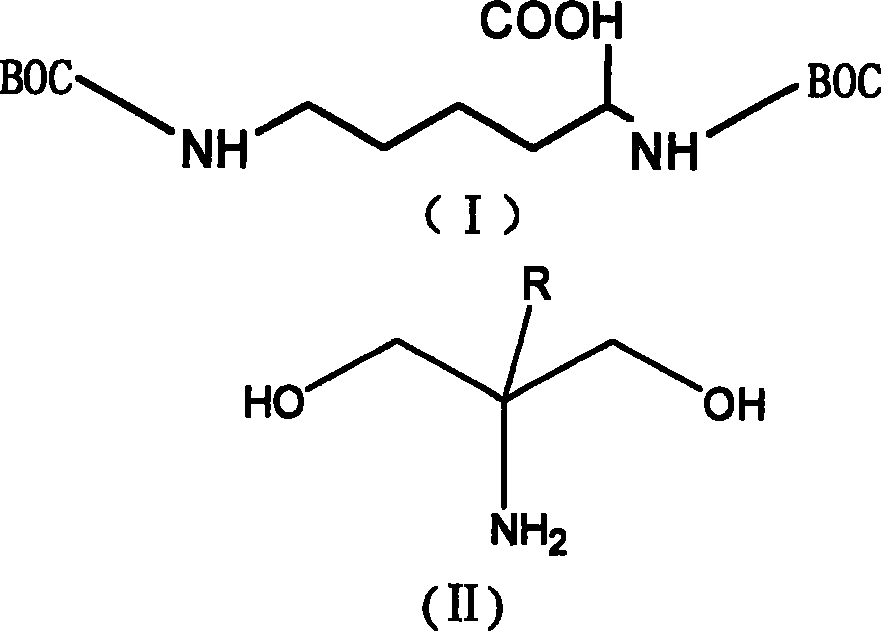
[0039] The synthetic route is shown in the figure above.
[0040] Step 1): 4.85 grams (14mmol) of α, omega-bishydroxy tert-butoxycarbonate base lysine, 1.58 grams (15mmol) of 2-amino-2-methyl-1,3-propanediol were dissolved in 30ml N , in a mixed solvent of N-dimethylacetamide and 40ml tetrahydrofuran, add 2.94 grams (21.78 mmol) of 1-hydroxybenzotriazole, cool to 0°C with an ice bath, and then add N, N'-bicyclic 3 grams (14 mmol) of hexylcarbodiimide carbon, reacted for 24 hours, removed the precipitate by suction filtration, added 100ml of dilute hydrochloric acid, extracted with ethyl acetate, washed the organic layer with saturated sodium carbonate solution, saturated saline, and water five times, and reduced pressure Concentrate and perform silica gel column chromatography (chloroform:methanol=50:1) to obtain 4.06 g of pure lysine protected with dihydroxy tert-butoxycarbonate groups in the side chain (yield: 67%).
[0041] obtained pure 1 H-NMR (CD3Cl) δppm, 1.27 (m, 2H)...
Embodiment 2
[0051] The synthetic route is shown in the figure above.
[0052] Step 1): 4.85 grams (14 mmol) of α, ω-bishydroxy tert-butoxycarbonate base lysine, 1.58 grams (15 mmol) of 2-amino-2-methyl-1,3-propanediol, dissolved in 30 ml di In the methyl sulfoxide solvent, add 5.73 grams (16.8 mmol) of N-hydroxysuccinimide, cool to 0 ° C with an ice bath, then add 1.85 grams (14.7 mmol), reacted for 36h, removed the precipitate by suction filtration, added 100ml of dilute hydrochloric acid, extracted with ethyl acetate, and the organic layer was washed five times with saturated sodium carbonate solution, saturated brine, and water, concentrated under reduced pressure, and silica gel column chromatography (chloroform: Methanol=50:1), to obtain 4.06 g of pure lysine protected with dihydroxy tert-butoxycarbonate group in the side chain (yield: 67%).
[0053] obtained pure 1 H-NMR (CD3Cl) δppm, 1.27 (m, 2H), 1.43 (m, 23H), 1.80 (m, 2H), 3.12 (m, 2H), 3.70 (m, 3H), 3.89 (m, 2H), 4.01 (s, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com