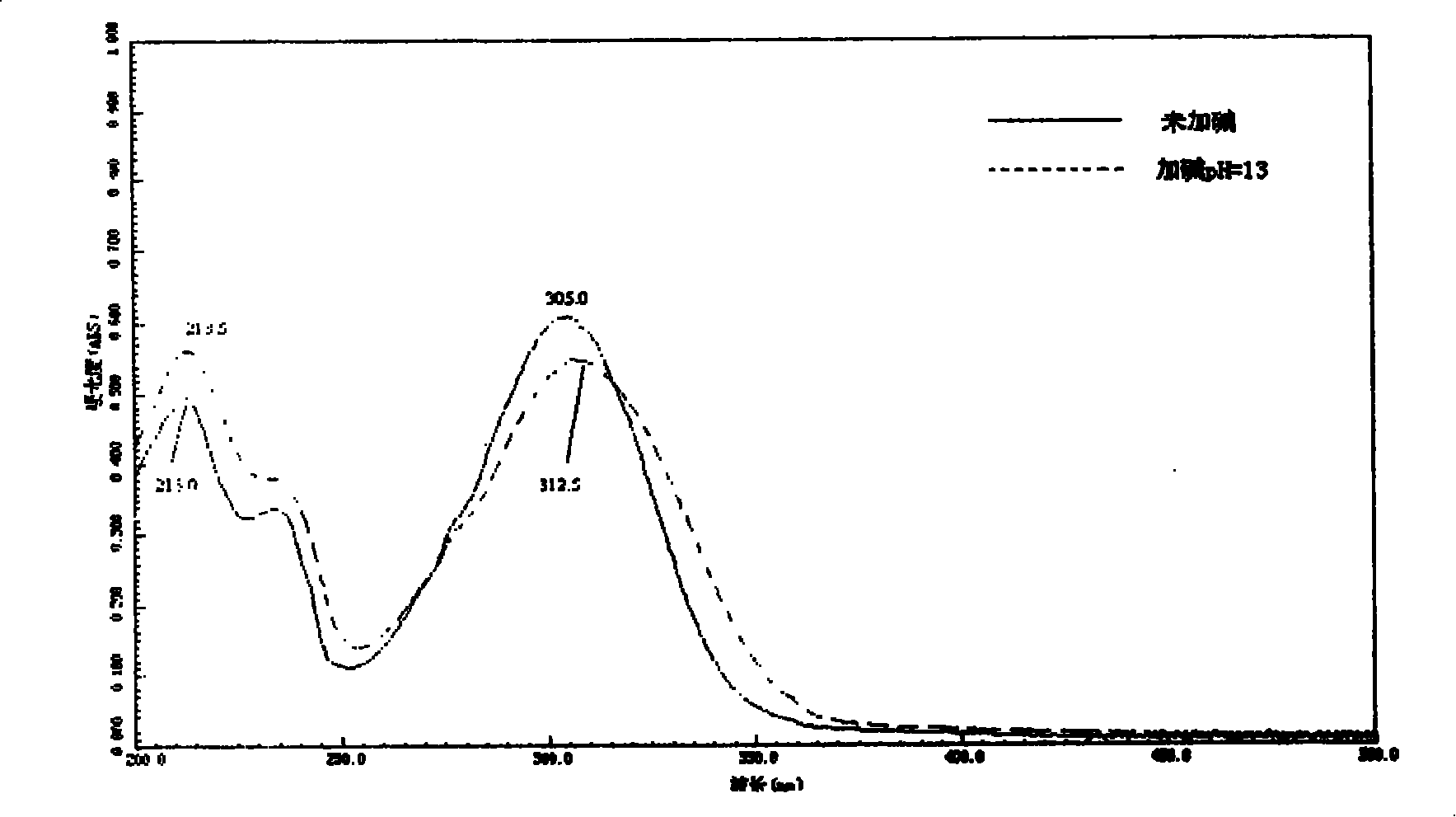
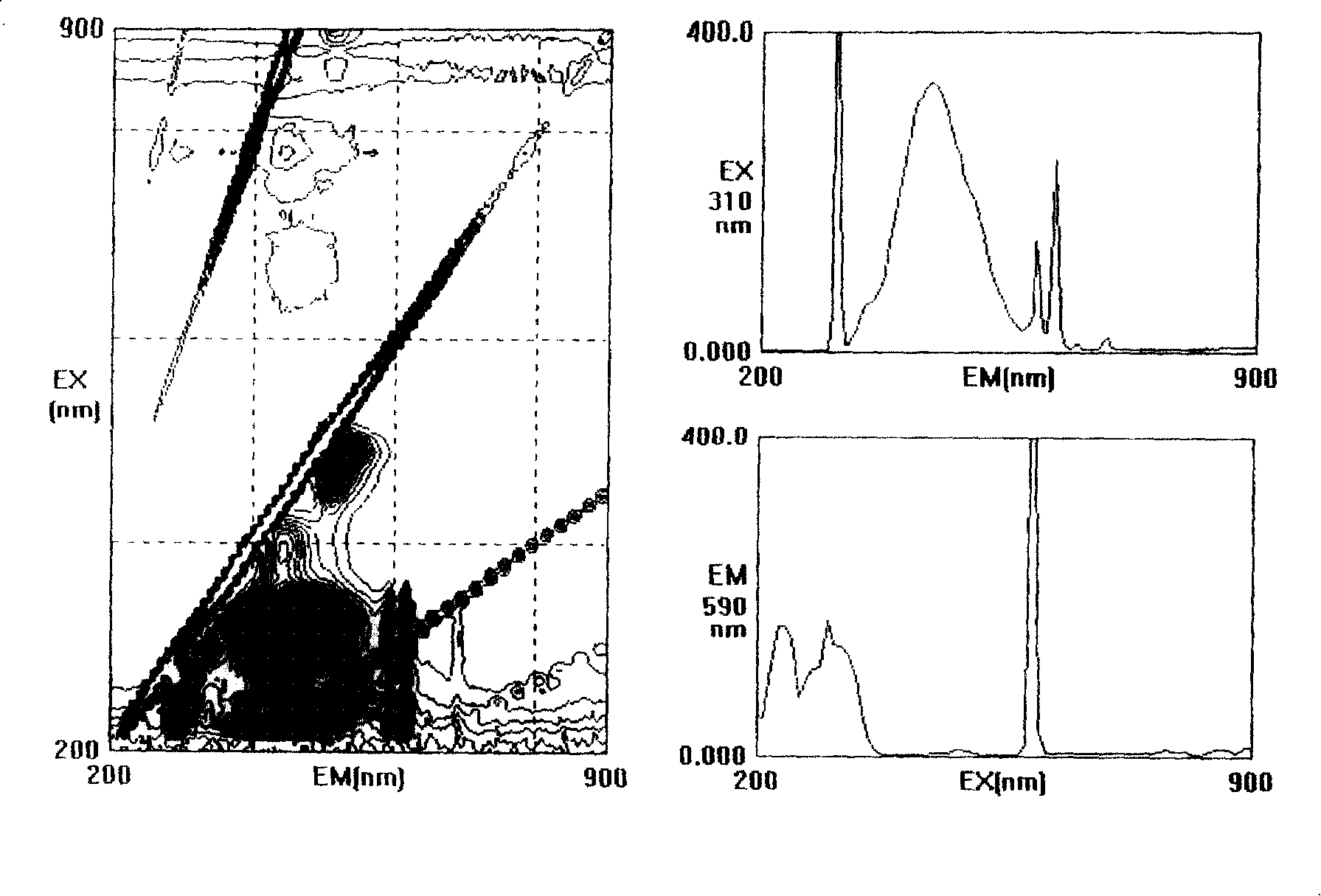
2-acetyl benzimidazolyl glycine-condensate Schiff base rare-earth complex and process for preparing same
A technology of acetylbenzimidazole glycine Schiff base and rare earth complexes, which is applied in the field of rare earth complexes and their preparation, can solve the problems of prolonging the reaction time, failing to reach the yield, serious pollution in the reaction process, etc. The process is simple, the synthesis method is improved, and the effect of excellent luminescence characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthetic method of embodiment 1 precursor 2-acetyl benzimidazole
[0034] Step 1: Synthesis of 2-(1'-hydroxyethyl)benzimidazole:
[0035]
[0036] In a 100ml three-neck flask, dissolve 10g of o-phenylenediamine in 25ml of 4mol / L HCl, stir until completely dissolved, adjust the pH to 2 with HCl, and then add 0.1g of catalyst FeCl 3 ·6H 2 O and 13ml lactic acid, heated to reflux for 4 hours, cooled, slowly added solid Na 2 CO 3 Neutralize the powder and stir vigorously until the pH is close to 7, then add 20% Na 2 CO 3 Adjust until the solution changes from black-red to yellow-green, and a large amount of precipitates are formed, which is left to stand and filtered with suction to obtain the crude product. Recrystallize directly with water, add activated carbon for decolorization, filter while hot, and precipitate 13.5 g of white crystals after cooling, with a yield of 80% and a melting point of 180.4-181.0°C (literature value of 177-179°C).
[0037] Step 2:...
Embodiment 2
[0039] The preparation of embodiment 2 ligand
[0040] Glycine (0.7507g, 0.01mol) and KOH (0.56g, 0.01mol) were dissolved in 25mL of methanol, stirred for about 15 minutes until dissolved, filtered, and 2-acetylbenzo Imidazole (1.602g, 0.01mol) in 20mL of methanol solution, after reflux for 5-14 hours, the color of the solution changed from yellow to dark brown red, part of the methanol was evaporated, cooled, a precipitate formed, suction filtered, washed with methanol and chloroform respectively, Dry it under infrared light, place it in a concentrated sulfuric acid desiccator to obtain a khaki powder.
[0041] 1 H NMR hydrogen spectrum (400MHz, CD 3 OD): δ7.6(q, 2H, a Coupling peak on H-Ar), δ7.3(q, 2H, b Coupling peak on H-Ar), δ5.311 (d, 1H, H-N), δ2.582 (s, 2H, -CH 2 -), δ1.652(s, 3H, CH 3 ).
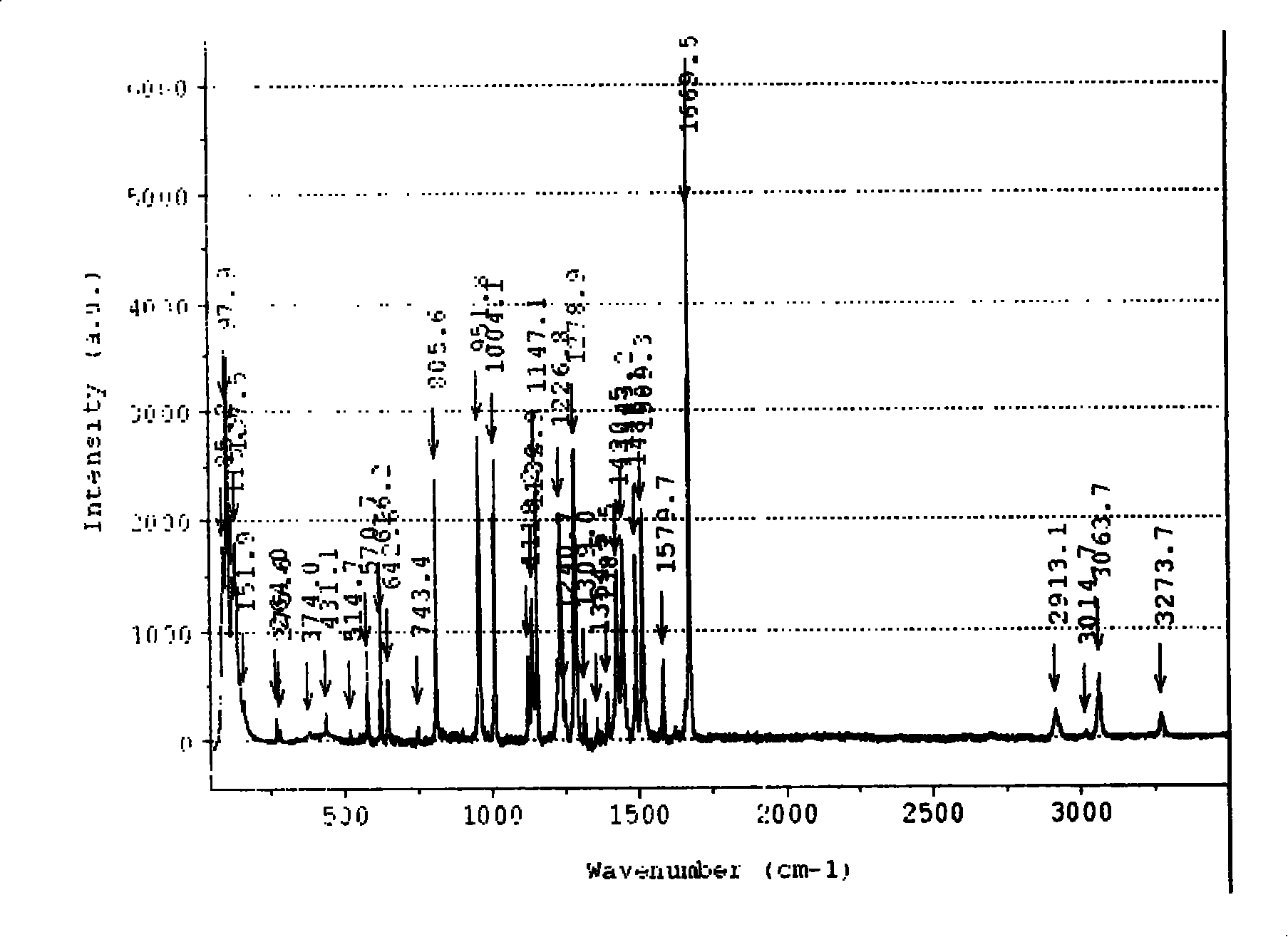
[0042] Infrared (cm -1 )(KBr) 1643(C=N), 1570(v as COO - ), 1428 (v s COO - )
[0043] Raman (cm -1 ): 1644.76 (C=N), 1566.93 v as COO - ), 1436.76 (v s COO - ), ...
Embodiment 3
[0045] Embodiment 3 rare earth Pr complexes [Pr 2 L 3 (NO 3 ) 3 ·2CH 3 OH] Preparation
[0046] The rare earth nitrate Pr(NO 3 ) 3 ·6H 2 O (0.87g, 2mmol) in 25mL of methanol was slowly added dropwise to 30mL of hot methanol in which Schiff base (KL) ligand (0.79g, 3mmol) was dissolved in a stoichiometric ratio of 2:3 (rare earth ion: Schiff base ligand) In the solution, the reaction solution immediately became cloudy and earthy yellow. After reacting at 50°C for 3 hours, it was cooled and filtered with suction. After the precipitate was washed with hot methanol solution for several times, it was slowly dried in an oven at 50°C and placed in a concentrated sulfuric acid drier. Up to constant weight.
[0047] Infrared (cm -1 )(KBr): 1655(C=N), 1591(v as COO - ), 1437 (v s COO - ), 1384 (NO - 3 ), 1508 (NO - 3 ), 822 (NO - 3 ), 1316 (NO - 3 )
[0048] Raman (cm -1 ): 1639.40 (C=N), 1586.82 (v as COO - ), 1442.92 (v s COO - ), 1485.49 (NO - 3 ), 817.80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com