Magnetic separation enzymatic chemical luminous immune detection method of human thyroglobulin antibody
A thyroglobulin, enzymatic chemiluminescence technology, used in chemiluminescence/bioluminescence, analysis by chemical reaction of materials, measurement devices, etc., can solve health hazards, short shelf life, and unstable radioconjugates and other problems, to achieve the effect of high affinity, improved sensitivity, and reduced non-specific adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of secondary antibody enzyme-labeled reagent
[0042] Mix mouse anti-human immunoglobulin A, G, E, M, D, κ chain and λ chain monoclonal antibodies, and concentrate the concentration to 5mg / mL; add 20mmol / L activator N-succinimide 3-(2 -Pyridyldithiol) propionic acid [SPDP] dimethyl sulfoxide solution 20 μL, stand at room temperature for 45 minutes; remove the activator through a Sephadex G25 column, and collect the protein peak; add 500 μL 24 mg / mL dithiothreose to each ml of activated antibody solution Alcohol (DTT) 0.01mol / L PBS pH7.4 solution, after mixing, let stand at room temperature for 30 minutes; remove free dithiothreitol through Sephadex G25 column, and collect protein peaks.
[0043]Dissolve alkaline phosphatase in 2mmol / L EDTA 20mmol / L Tris-HCl pH8.0 solution, the concentration is 5mg / mL; add 0.10mg / mL Traunt reagent (sulfhydryl activating reagent) and let it stand at room temperature for 1 hour; remove free activator to collect prot...
Embodiment 2
[0046] Embodiment 2: the preparation of solid phase reagent
[0047] Activate ferric oxide microspheres with a diameter range of 0.1 μm with glutaraldehyde, mix at room temperature for 4 hours, wash with 0.01mol / L PBS pH7.4 buffer three times, and use this solution to suspend at a concentration of 50- 100mg / mL; then, add human thyroglobulin antigen 100μg to each milliliter suspension, mix and incubate at 37°C for 3-8 hours; use an equal volume of 0.01mol / L PBS 5%BSA pH7.4 ℃ blocking for 40 minutes; finally, wash three times with 0.5% BSA 0.02mol / L Tris-HCl pH8.0 buffer solution, and use this solution to prepare 8 mg / mL working solution.
Embodiment 3
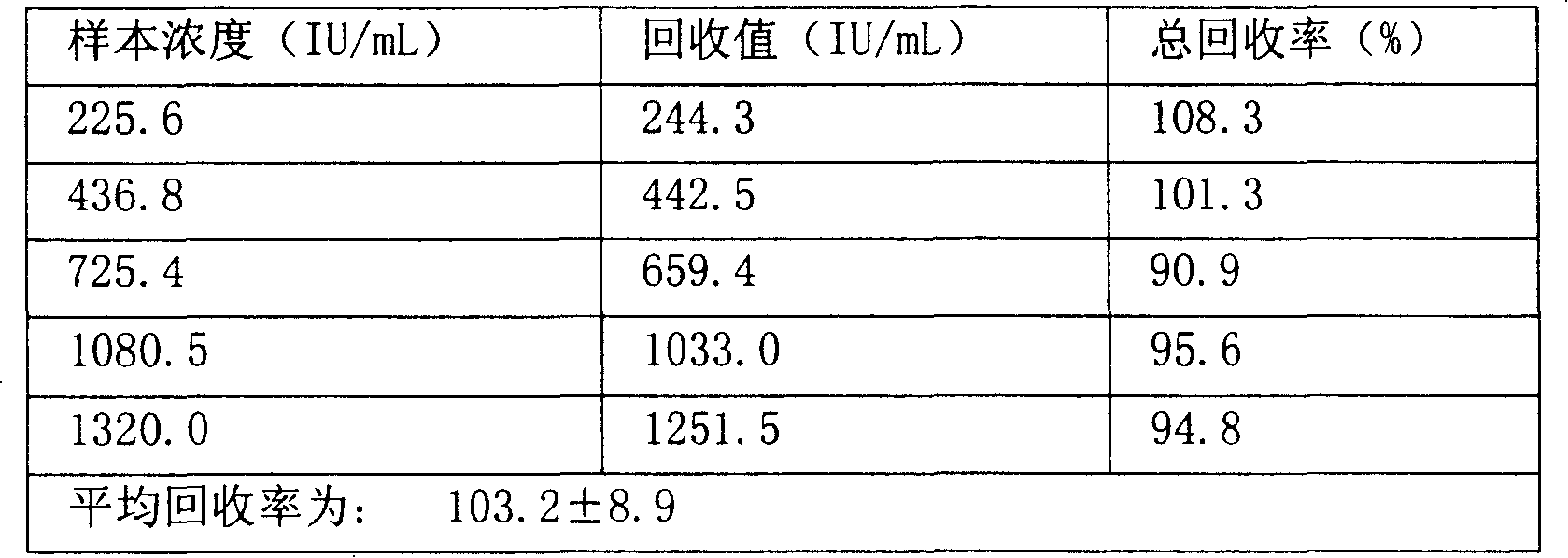
[0048] Example 3: Magnetic separation of human thyroglobulin antibody by enzymatic chemiluminescence immunoassay
[0049] Materials and Instruments
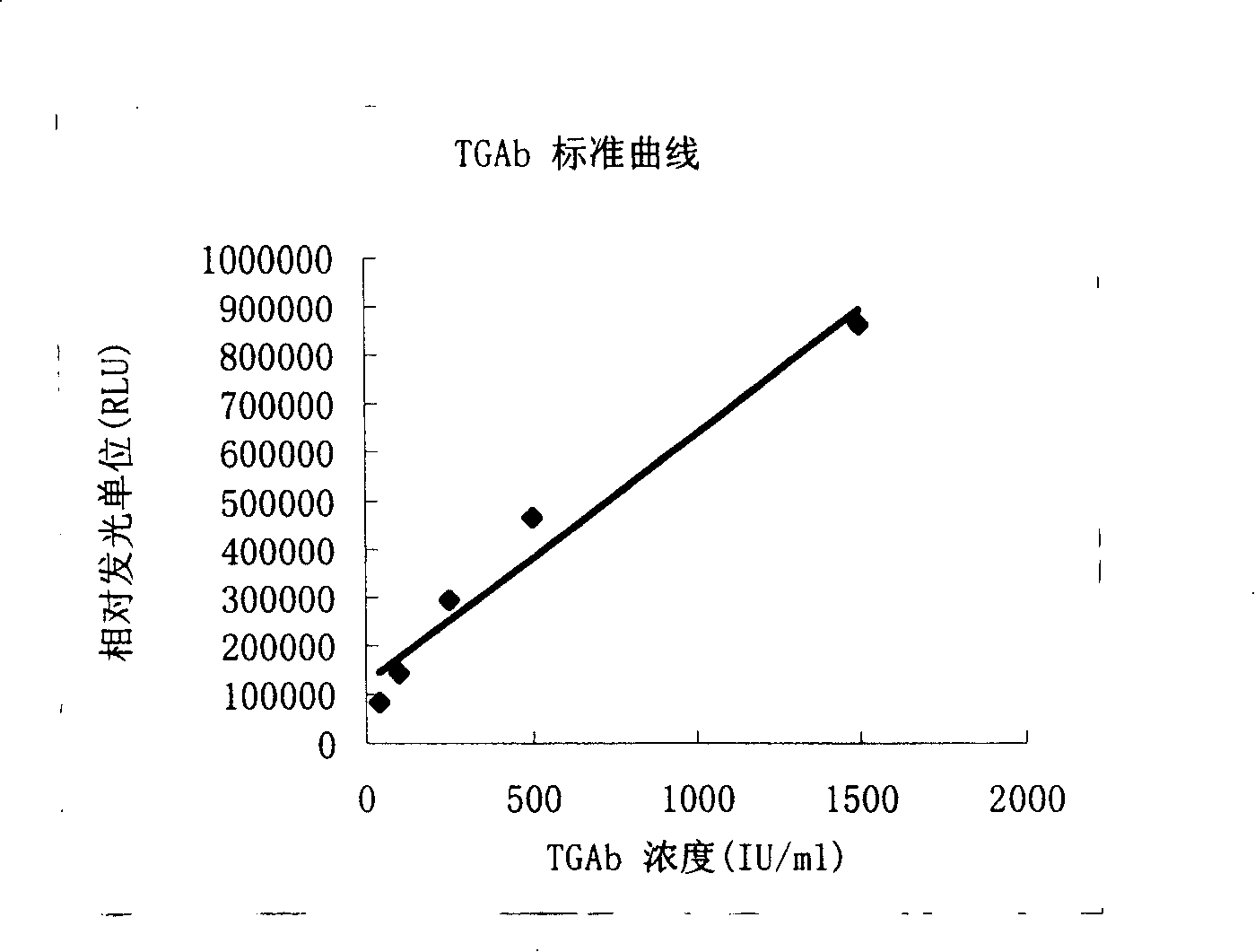
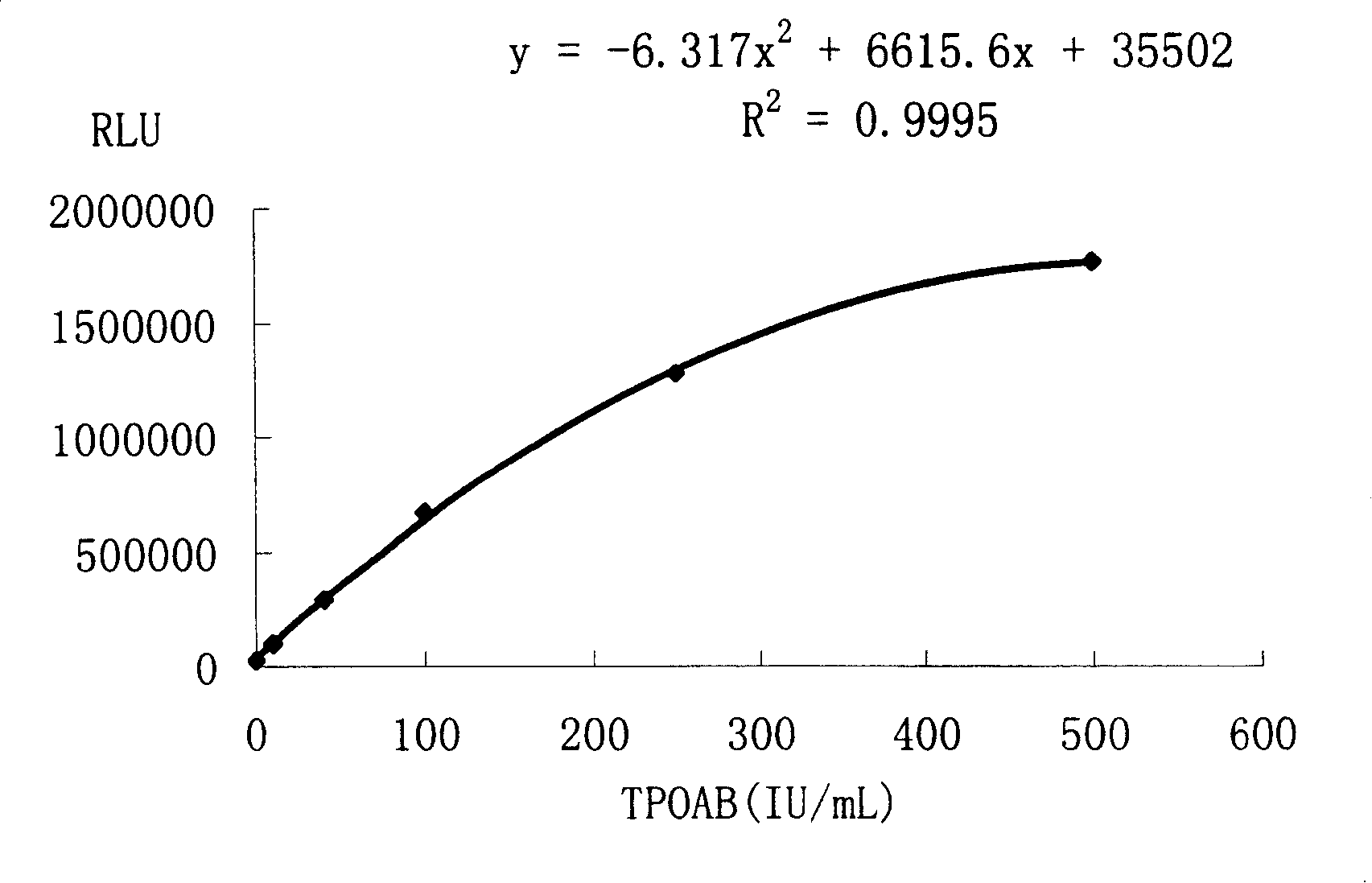
[0050] 1. Standard 0, 40, 100, 250, 500, 1500IU / mL (refer to WHO 1 ST IRP 66 / 387 standard)
[0051] 2. Solid-phase reagent: see Embodiment 2 for details.
[0052] 3. Secondary antibody enzyme-labeled reagent: see Embodiment 1 for details.
[0053] 4. Washing solution 100mL;
[0054] 5. Substrate solution: purchased from Applied Biosystems, USA
[0055] 6. The enhancer solution was purchased from Applied Biosystems, USA
[0056] 7. Sample diluent
[0057] 8. 100 serum samples from patients with thyroid autoimmune disease and 100 serum samples from normal people.
[0058] 9. BPCL chemiluminescence detector (manufactured by Institute of Biophysics, Chinese Academy of Sciences).
[0059] 10. Water bath (for 37°C warm bath).
[0060] Reagent preparation:
[0061] The preparation of the standard product is: 2g sodium chlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com