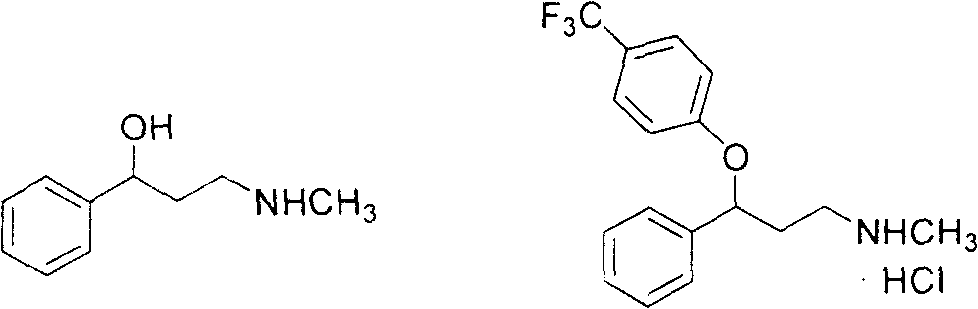
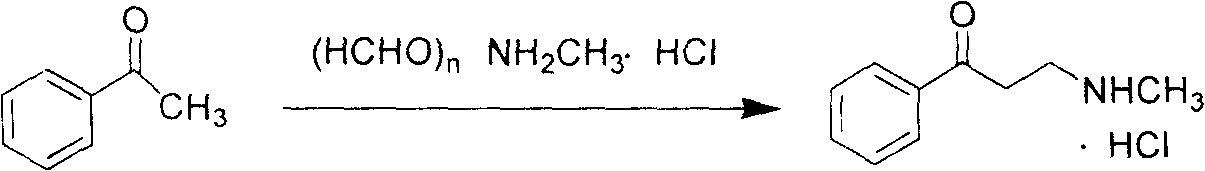3- methylamino-1-phenylpropanol preparation method
A technology of methylamino and phenylpropanol, which is applied in the field of synthesis of chemical drug intermediates, can solve the problems of high COD value wastewater, difficult solvent recovery, high production costs, etc., achieve less discharge of three wastes, reduce dehydroxylation side reactions, and production costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 120.0g of acetophenone, 30.0g of paraformaldehyde, and 67.5g of monomethylammonium hydrochloride (molar ratio 1:1:1) were added to a 100ml autoclave, and 500ml of ethanol was added to keep the reaction temperature at 60°C. Samples were taken every 2 hours for liquid phase detection, and the reaction was stopped until the difference in the content of 3-methylamino-1-propiophenone hydrochloride in two adjacent detection results was less than 5%. The volume of the reaction solution was concentrated by heating to about 1 / 3 of the original volume, cooled and crystallized, and filtered to obtain about 121 g of white crystal or slightly yellow solid 3-methylamino-1-propiophenone hydrochloride.
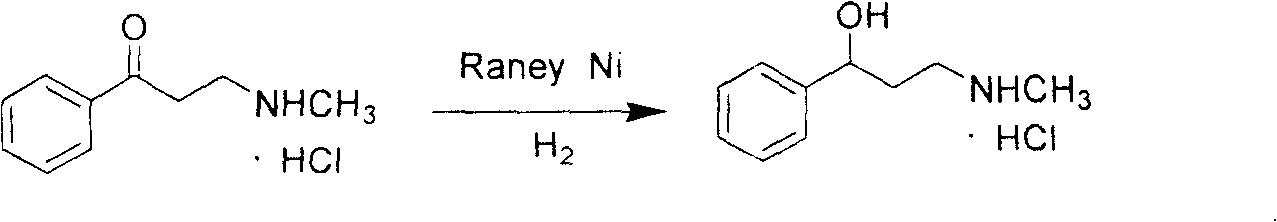
[0022] Add the 3-methylamino-1-propiophenone hydrochloride obtained above into a 1000mL stainless steel autoclave, add water until the solid is completely dissolved, add 6.1g of Raney nickel catalyst, first replace it with nitrogen for 3 times, then use 0.3MPa hydrogen Replace 3 times,...
Embodiment 2
[0024] Add 120.0g of acetophenone, 30.0g of paraformaldehyde, and 101.3g of monomethylammonium hydrochloride (molar ratio 1:1:1.5) into a 1000ml autoclave, and add 500ml of isopropanol to keep the reaction temperature at 90°C , take samples every 2 hours for liquid phase detection, the difference in the content of 3-methylamino-1-propiophenone hydrochloride in two adjacent detection results is less than 5%, and the volume of the heated and concentrated reaction solution is about 1 / 3 of the original volume , cooled and crystallized, and filtered to obtain about 162.7 g of white crystal or slightly yellow solid 3-methylamino-1-propiophenone hydrochloride.
[0025] Add the 3-methylamino-1-propiophenone hydrochloride obtained above into a 1000mL stainless steel autoclave, add water until the solid is completely dissolved, add 16.3g of Raney nickel catalyst, first replace it with nitrogen for 3 times, then use 1.5MPa hydrogen Replace 3 times, control the temperature at 80°C under s...
Embodiment 3
[0027] 120.0g of acetophenone, 45.0g of paraformaldehyde, and 67.5g of monomethylammonium hydrochloride (molar ratio 1:1.5:1) were added to a 1000ml autoclave, and 500ml of ethanol was added to keep the reaction temperature at 90°C. Sampling was carried out at intervals of 2 hours for liquid phase detection, and when the difference in the content of 3-methylamino-1-propiophenone hydrochloride between two adjacent detection results was less than 5%, the reaction liquid was cooled. The volume of the reaction solution was concentrated by heating to about 1 / 3 of the original volume, cooled and crystallized, and filtered to obtain about 170.2 g of white crystal or slightly yellow solid 3-methylamino-1-propiophenone hydrochloride.
[0028] Add the 3-methylamino-1-propiophenone hydrochloride obtained above into a 1000ml stainless steel autoclave, add water until the solid is completely dissolved, add 8.5g of Raney nickel catalyst, and use N 2 Substitute twice, then replace twice with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com