Drug injection of recombinant human erythropoietin
A technology for erythropoietin and injections, which is applied in the field of drug injections of recombinant human erythropoietin, can solve problems such as complex production processes, and achieve the effects of simple formula, reduced drug auxiliary components, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare 3000 recombinant human erythropoietin injections of 3000IU / ml /
[0034] 1. Formula composition:
[0035] Recombinant Human Erythropoietin 3000IU / ml
[0036] Polysorbate 80 0.03mg / ml
[0037] Sodium dihydrogen phosphate dihydrate 2.22mg / ml
[0038] Disodium hydrogen phosphate dodecahydrate 6.45mg / ml
[0039] Sodium chloride 8.7mg / ml
[0040] Water for injection up to 3000ml
[0041] 2. Preparation:
[0042] Measure the medicinal amount of recombinant human erythropoietin stock solution, 90mg polysorbate 80, add 18mM sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution, containing 26.1g sodium chloride to 3000ml, shake well.
[0043] 3. Packing;
[0044] Sterile filter the prepared solution into sterile containers. Then it is divided into medical glass containers, 1 ml is added to each bottle, and filled to make 3000 IU / ml recombinant human erythropoietin injection.
Embodiment 2
[0046] Preparation of 3000 recombinant human erythropoietin injections of 6000IU / ml /
[0047] 1. Formula composition:
[0048] Recombinant Human Erythropoietin 6000IU / ml
[0049] Polysorbate 80 0.03mg / ml
[0050] Sodium dihydrogen phosphate monohydrate 1.68mg / ml
[0051] Disodium hydrogen phosphate 2.56mg / ml
[0052] Sodium chloride 8.7mg / ml
[0053] Water for injection up to 3000ml
[0054] 2. Preparation:
[0055] Measure the medicinal amount of recombinant human erythropoietin stock solution, 90mg polysorbate 80, add 18mM sodium dihydrogen phosphate / disodium hydrogen phosphate buffer solution, containing 26.1g sodium chloride to 3000ml, shake well.
[0056] 3. Packing;
[0057] Sterile filter the prepared solution into sterile containers. Then it is divided into medical glass containers, 1 ml is added to each bottle, and filled to make 6000 IU / ml recombinant human erythropoietin injection.
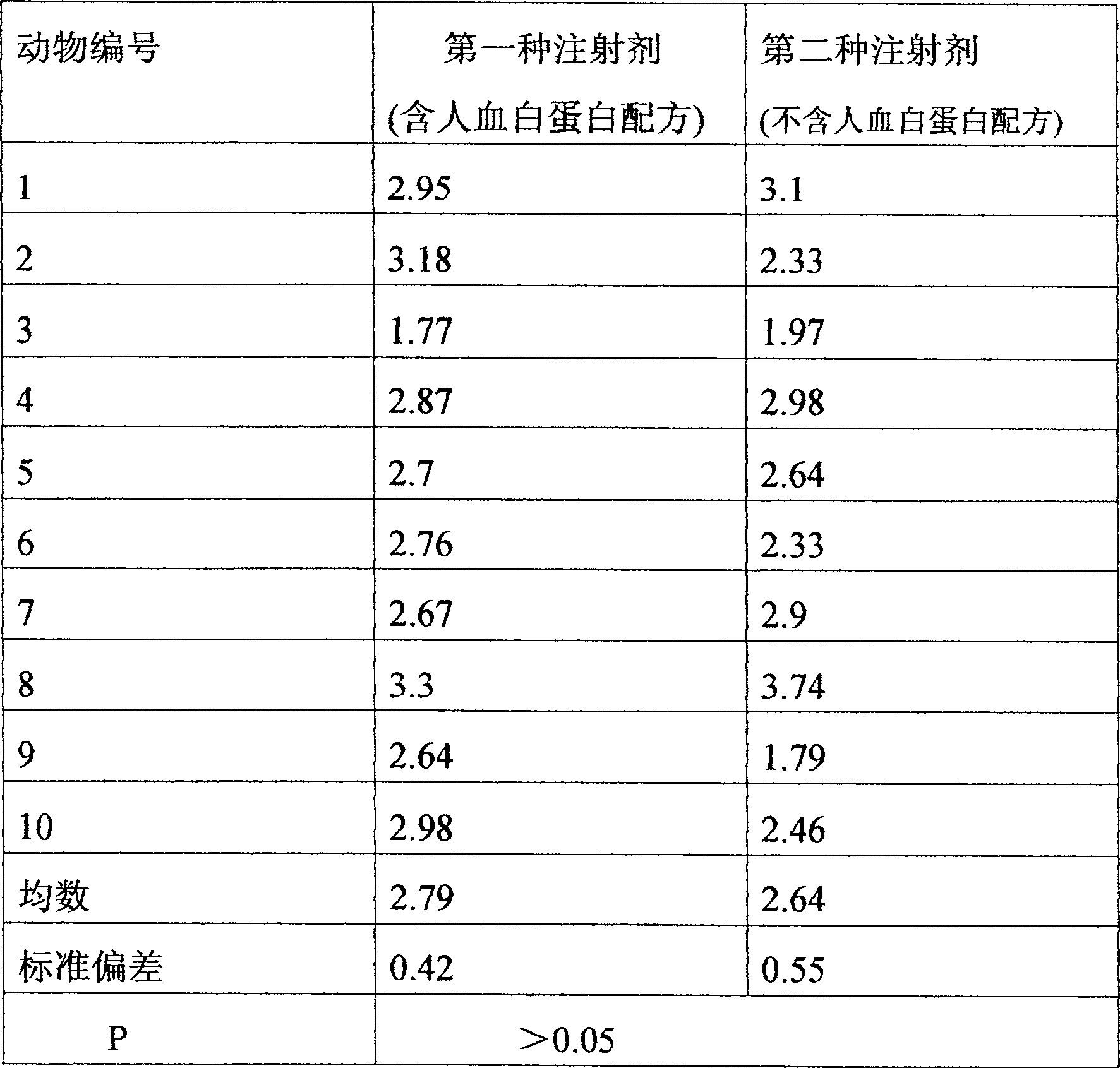
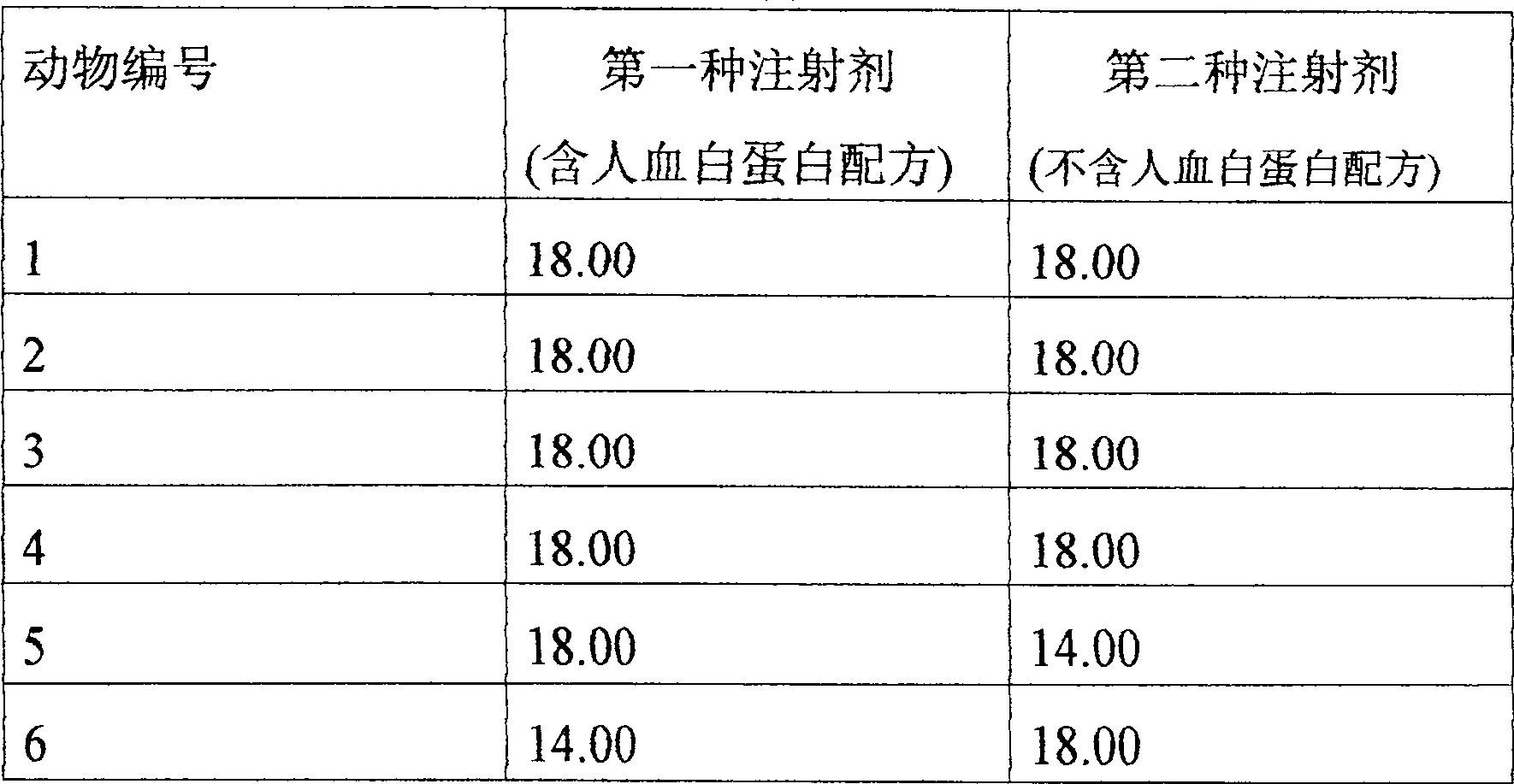
[0058]The injection of the present invention exhibits pharmacokinetic prop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com