Chemical synthesizing process of beta-butyrolactone with lactide copolymer
A technology of chemical synthesis and lactide, applied in the field of polymer synthesis, can solve the problems of poor mechanical properties, low impact strength, difficult processing, etc., and achieve the effects of improved crystallinity and processing performance, and narrow relative molecular mass distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
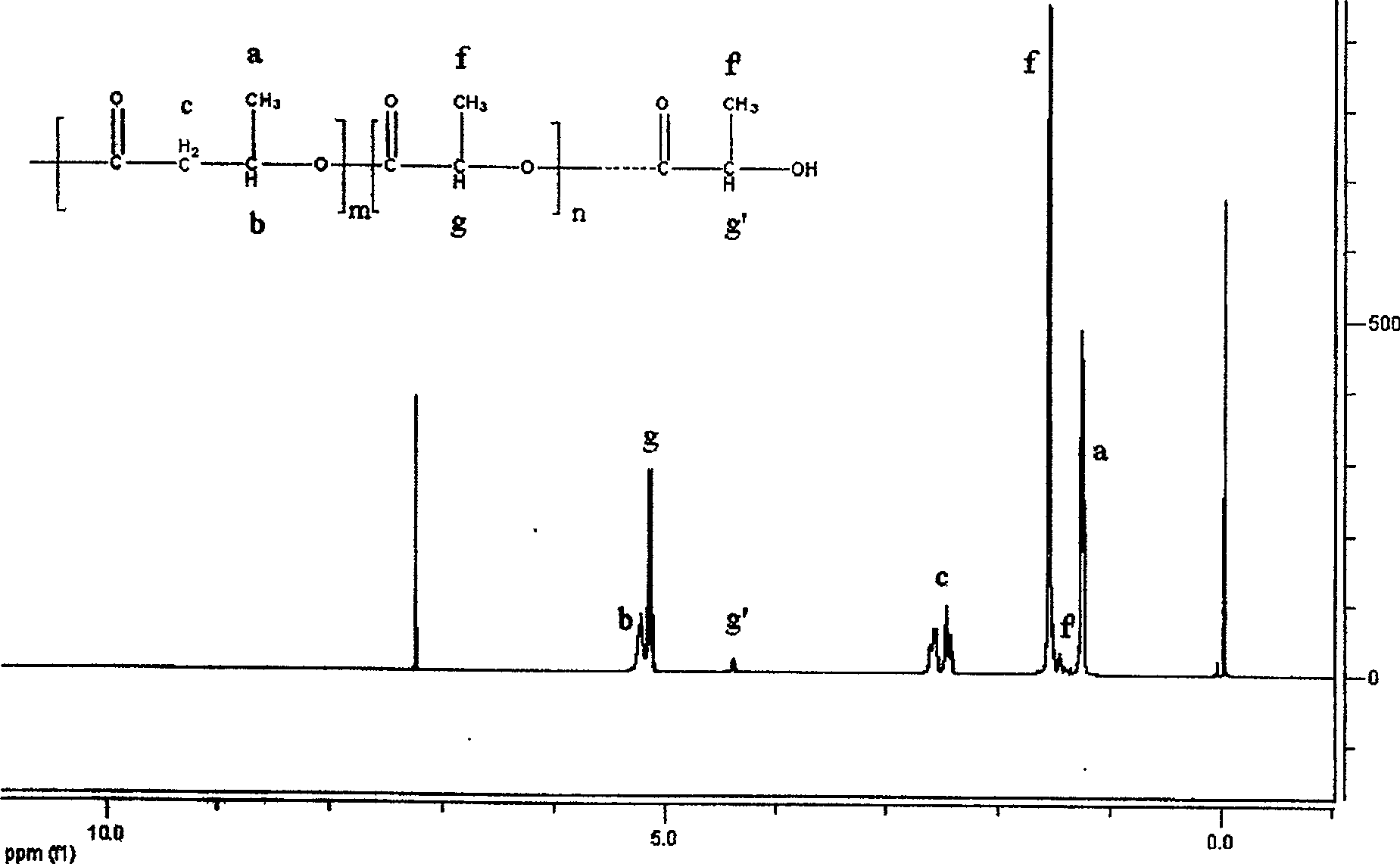
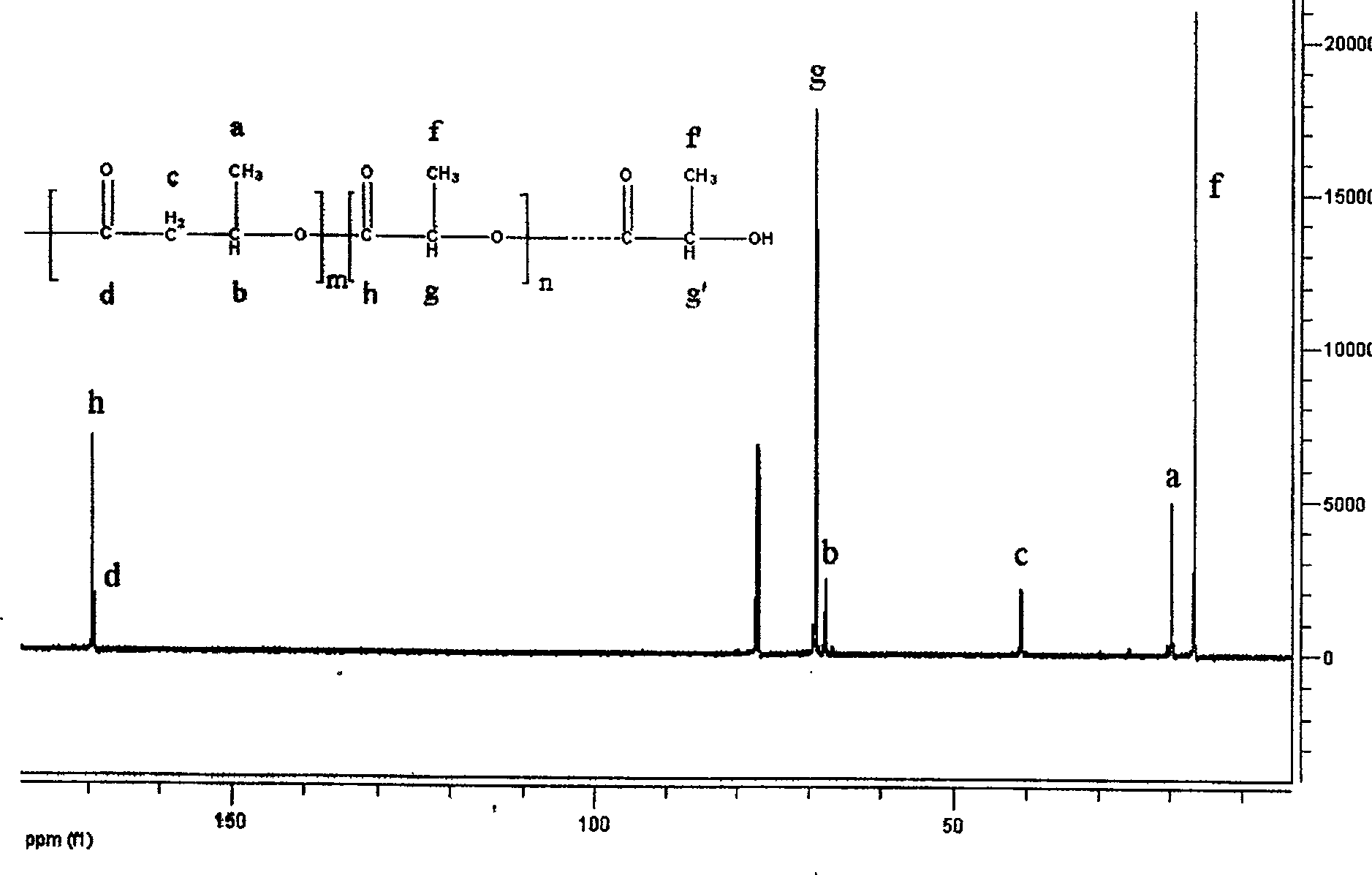
[0021] (1) the catalyst dibutyl tin dichloride of 0.0033g is packed in the reaction vessel and vacuum-dried for 48 hours, (2) the β-butyrolactone of 0.33g is added in the reaction vessel, (3) the L-butyrolactone of 33g is added Lactide was added to the reaction vessel and the reaction vessel was sealed. (4) Reacting at 120° C. for 48 hours under magnetic stirring to obtain a colorless transparent solid. (5) The product obtained in step (4) was dissolved in dichloromethane to obtain solution A. Add A dropwise to excess anhydrous methanol to obtain a white precipitate B, wash and dry the obtained precipitate B to obtain a copolymer of β-butyrolactone and L-lactide, which 1 The H-NMR spectrum is attached figure 1 , 1.25ppm, 2.47ppm, 2.59ppm, and 5.23ppm belong to the characteristic peaks of the poly 3-hydroxybutyrate segment, and 1.56ppm and 5.15ppm belong to the characteristic peaks of the polylactic acid segment. 13 C-NMR attached figure 2 , 19.8ppm, 40.7ppm, 67.7ppm and 1...
Embodiment 2
[0024] (1) the catalyst dibutyl tin dichloride of 0.33g is packed in the reaction vessel, (2) the β-butyrolactone of 33g is added in the reaction vessel, (3) the D of 0.33g, L-lactate The ester was added to the reaction vessel and the reaction vessel was sealed. (4) React at 40° C. for 48 hours under stirring to obtain a colorless transparent solid. (5) The product obtained in step (4) was dissolved in dichloromethane to obtain solution A. Add A dropwise to excess anhydrous methanol to obtain a white precipitate B, wash and dry the obtained precipitate B to obtain a copolymer of β-butyrolactone and L-lactide, the number-average relative molecular mass of the copolymer is 3000.
[0025] In this example, the catalyst can also be one of dibutyltin oxide, dioctyltin oxide and dimethyl tin oxide, triethylaluminum / water, diethylzinc / water, porphyrin aluminum.
Embodiment 3
[0027] (1) the catalyst dibutyltin dichloride of 0.0033g is packed in the reaction vessel and vacuum-dried for 24 hours, (2) the β-butyrolactone of 0.33g is added in the reaction vessel, (3) the L of 0.55g - Lactide is added to the reaction vessel and the reaction vessel is sealed. (4) Reacting at 40° C. for 720 hours under stirring to obtain a colorless transparent solid. (5) The product obtained in step (4) was dissolved in dichloromethane to obtain solution A. Add A dropwise to excess anhydrous methanol to obtain a white precipitate B, wash and dry the obtained precipitate B to obtain a copolymer of β-butyrolactone and L-lactide, the number-average relative molecular mass of the copolymer is 12000.
[0028] In this example, the catalyst can also be one of dibutyltin oxide, dioctyltin oxide and dimethyl tin oxide, triethylaluminum / water, diethylzinc / water, porphyrin aluminum.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com