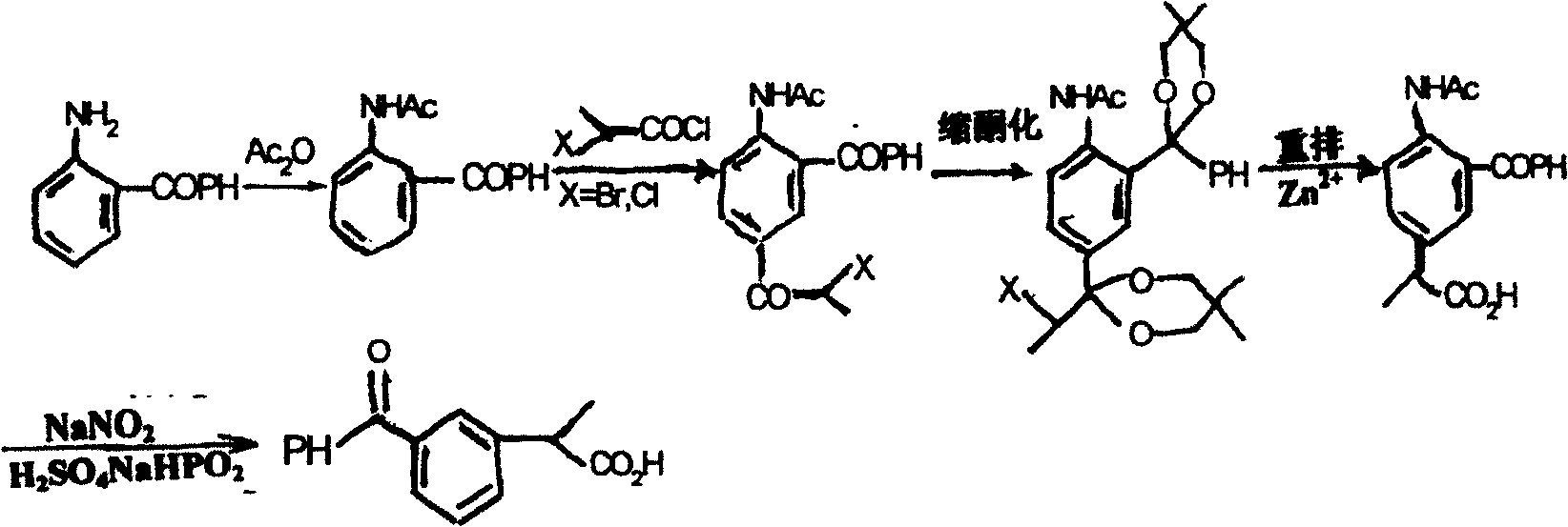
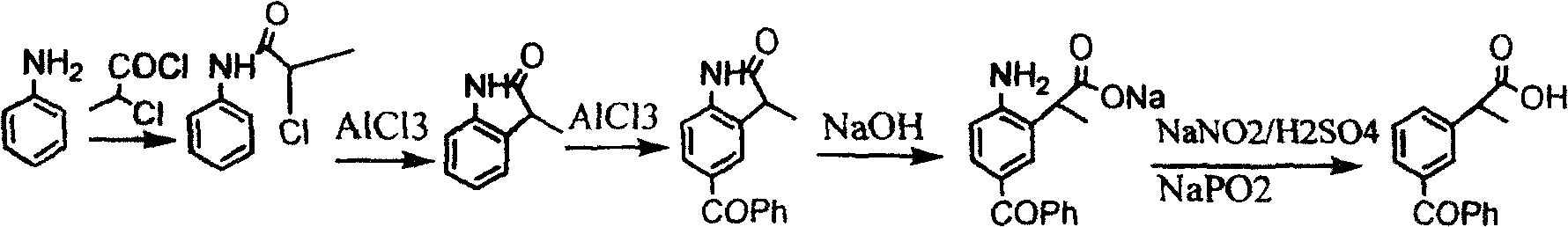
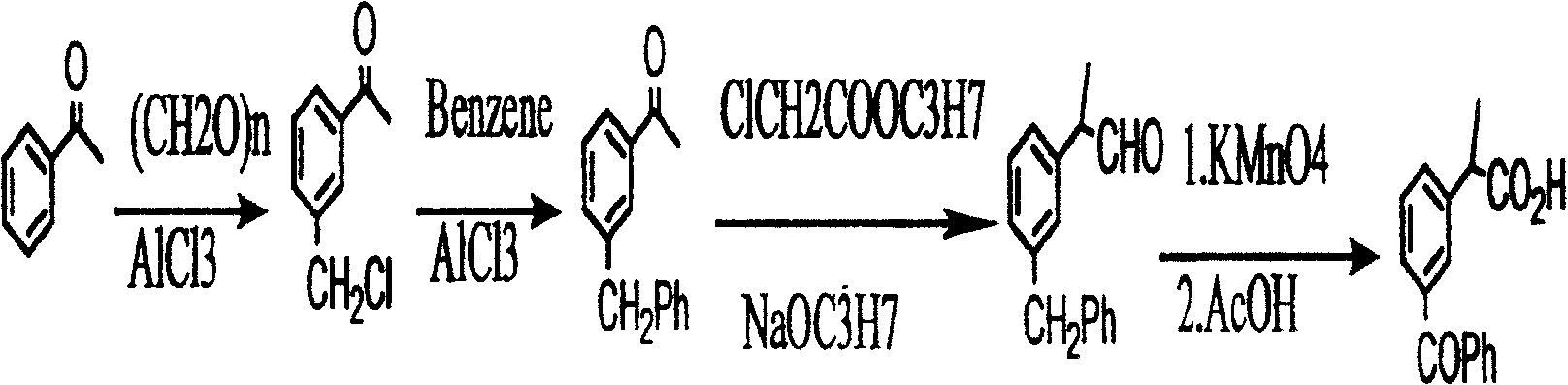Method for synthesizing ketoprofen by using ethylbenzene as raw material
A technology of ketoprofen and ethylbenzene, which is applied in the field of preparation of organic drugs, can solve problems such as low total yield, unfavorable environmental protection, and large fire accidents, and achieve the effects of reducing reaction steps, avoiding treatment difficulties, and reducing waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0030] 1. Synthesis of ortho (para) nitroethylbenzene:
[0031] Add 1mol of ethylbenzene into the reaction flask, add mixed acid (0.5molHNO3 / 0.5molH2SO4) dropwise at 31-35°C, then react at 40-50°C for 1 hour, stand for stratification, the obtained oil layer is washed with 100ml of water, 100ml of sodium bicarbonate After the neutralization of the aqueous solution, the excess ethylbenzene was recovered under reduced pressure, and the residue was a mixture of o-(p-)nitroethylbenzene, wherein about 33% of the ortho-position and about 66% of the p-position were transferred to the next step for carboxylation without separation and purification. The reaction is the synthesis reaction of sodium 2-[o-(p-)nitrophenyl]propionate.
[0032] 2. The synthetic reaction of 2-[o (p-) nitrophenyl] sodium propionate:
[0033] Add 1 mol of mixed nitroethylbenzene, 10 mol of DMF, and 2 mol of potassium carbonate to the autoclave, add dry carbon dioxide to 1.0 Mpa, heat and stir, react at 90-100 °...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com