Typhoid, paratyphoid envelope protein vaccine
A technology for outer membrane protein and paratyphoid fever, applied in the field of vaccines, can solve the problems of irregular stability of live attenuated vaccines, limited usage, and unsatisfactory effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
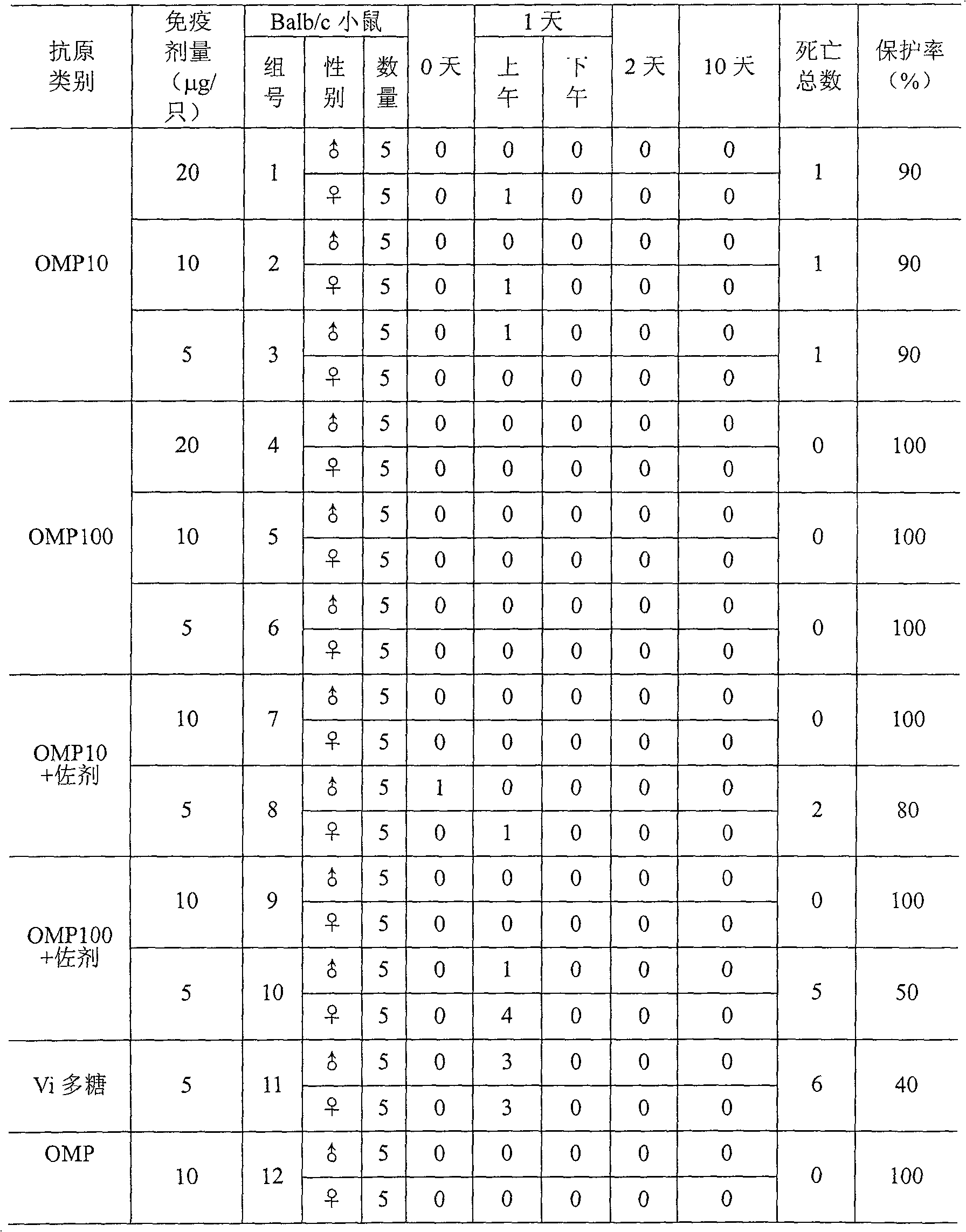
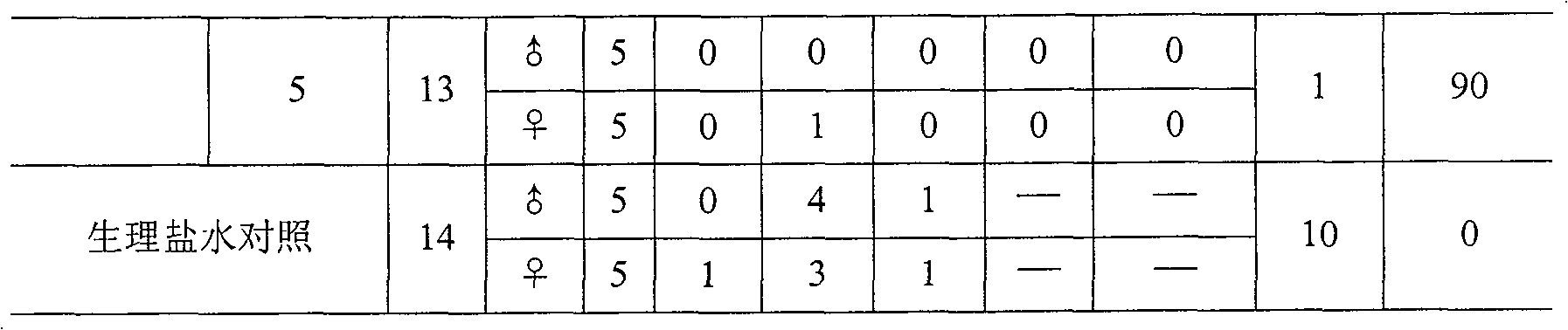
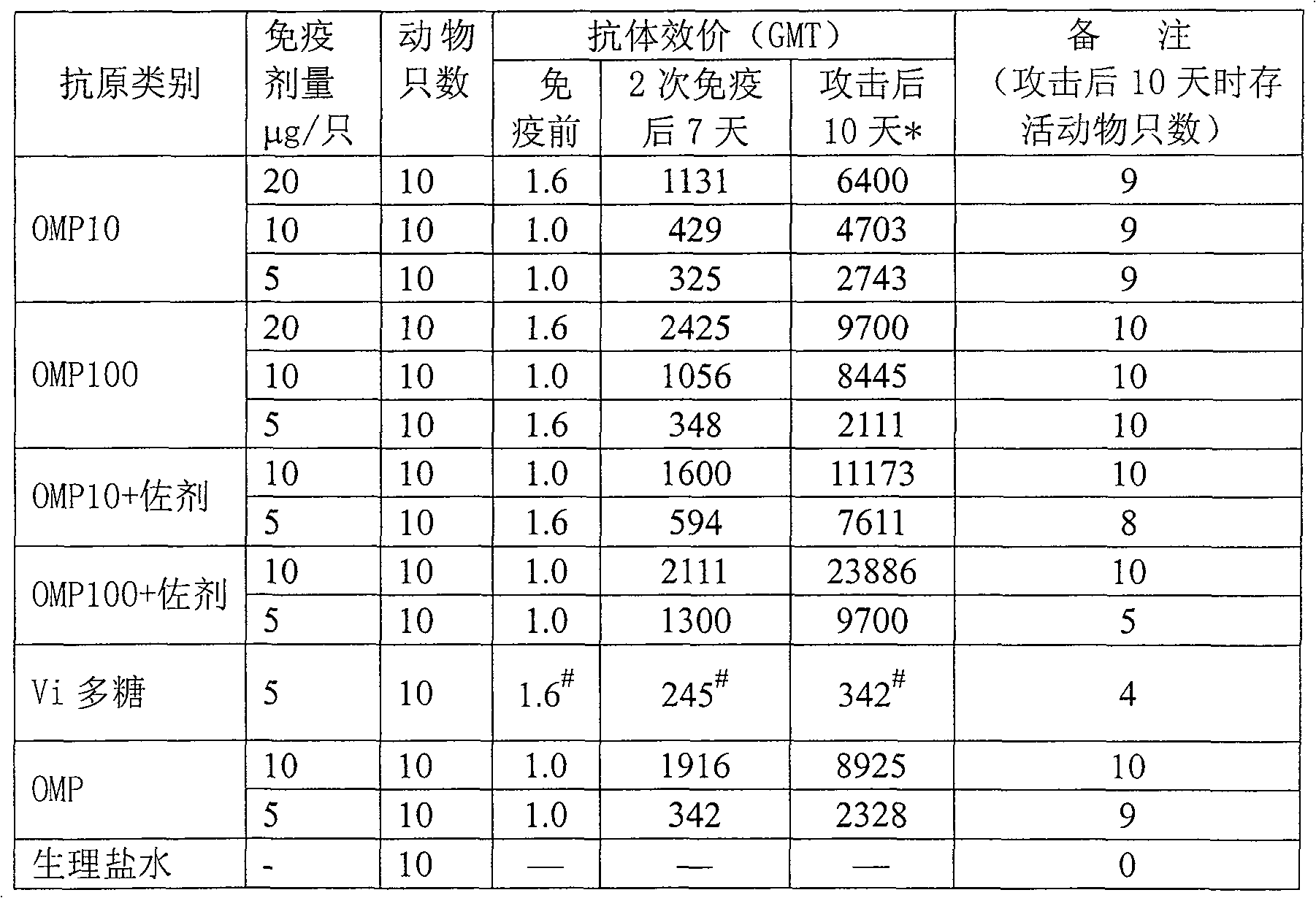
Embodiment 1
[0047] The preparation method of Bacillus typhi outer membrane protein:
[0048] Open typhoid strains, inoculate in semi-solid medium, then inoculate in 3L seed medium, place the culture bottle on a constant temperature shaker at 37°C, shake at 200 rpm, and cultivate until A 650 = 0.6~1.0, transfer to a 50L automatic bioreactor containing 30L medium, cultivate until just after the logarithmic growth phase (about 8~12 hours), add formaldehyde solution with a final concentration of 1% to sterilize 1 hours, 4 ° C, 11000 rpm centrifugation, typhoid bacteria (about 200 ~ 300 grams of wet weight), add 2000 ml of 100mM TRIS-HCl (pH 7.5 ~ 8.5, containing 20mM EDTA) buffer to dissolve, electric mixer stirring, Rotate at about 500 rpm, stir for 30 minutes to fully suspend the bacteria, then add 500 ml of 2.5% sodium deoxycholate (DOC) solution, adjust the rotating speed to about 250-300 rpm, stir at 4°C for 24-72 Hour.
[0049] Take out the typhoid bacterial suspension from the 4°C fr...
Embodiment 2
[0057] Preparation method of Bacillus paratyphi A outer membrane protein
[0058] Open the strains of paratyphoid A, inoculate in semi-solid medium, then inoculate in 3L seed medium, place the culture bottle on a constant temperature shaker at 37°C, 200 rpm, and cultivate to A 650 = 0.6 to 0.8, transfer to a 50L fully automatic bioreactor containing 30L medium, cultivate until just after the logarithmic growth phase (about 8 to 14 hours), add a final concentration of 1.5% formaldehyde solution to sterilize 1 Centrifuge at 4°C and 11,000 rpm for 1 hour to obtain typhoid thallus (about 200-250 g wet weight), add 2000 ml of 100 mM TRIS-HCl (pH 7.5-8.5, containing 20 mM EDTA) buffer to dissolve, stir with an electric mixer, Rotate at about 500 rpm, stir for 30 minutes, then add 500 ml of 2.5% Zwittergen-3-14 solution, adjust the rotating speed to about 250-300 rpm, and stir at 4°C for 24-72 hours.
[0059] Take out the bacterial cell suspension from the 4°C freezer, centrifuge at...
Embodiment 3
[0063] Preparation method of Bacillus paratyphi b outer membrane protein
[0064] Open the strain of paratyphoid B, inoculate it in a semi-solid medium, then inoculate it in a 3L seed medium, place the culture bottle on a constant temperature shaker at 37°C, 200 rpm, and cultivate it until A 650 = 0.4 to 0.8, transfer to a 50L fully automatic bioreactor containing 30L medium, cultivate until just after reaching the logarithmic growth phase (about 8 to 14 hours), add formaldehyde solution with a final concentration of 1.5% to sterilize 1 hours, 4 ° C, 11000 rpm centrifugation, typhoid bacteria (about 200 ~ 300 grams of wet weight), add 2000 ml of 100mM TRIS-HCl (pH 7.5 ~ 8.5, containing 20mM EDTA) buffer to dissolve, electric mixer stirring, Rotate at about 500 rpm, stir for 30 minutes, then add 500 ml of 2.5% sodium deoxycholate (DOC) solution, adjust the rotating speed to about 250-300 rpm, and stir at 4°C for 48-72 hours. Take out the bacterial cell suspension from the 4°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com