Thermophilic esterase/phosphatidase gene, engineering bacteria, enzyme and use thereof
A technology of phospholipase and thermophilic ester, which is applied in the fields of genetic engineering, application, plant gene improvement, etc., can solve the problems of poor heat resistance of porcine pancreatic phospholipase, production efficiency and cost of degumming process not reaching the optimum level, etc., to speed up Kinetic response, low requirement standard, strong resistance to high temperature and chemical substance denaturation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
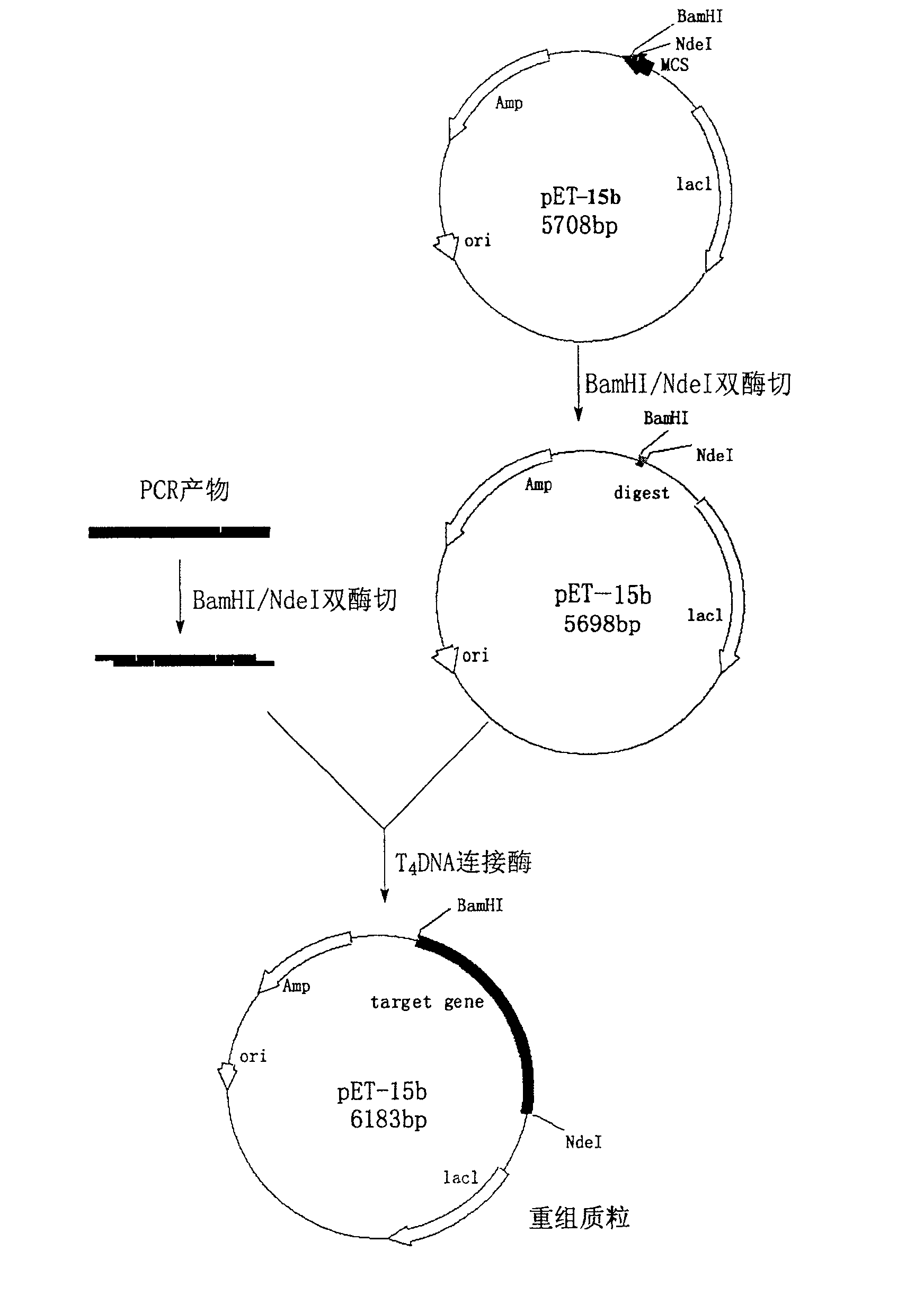
[0035] Embodiment 1: Construction of thermophilic enzyme engineering bacteria and its enzyme expression
[0036] (1) The cultivation of the thermophilic archaea Aeropyrum pernix K1 and the extraction of chromosomal DNA.
[0037] Take 37.4g Bacto marine broth 2216 (Difco) (culture medium) and dissolve it in 990ml distilled water, and sterilize with moist heat; take 1.0g Na 2 S 2 o 3 ·5H 2 O (nutrient salt) was dissolved in 10ml of distilled water, sterilized by 0.45m filter membrane and added to the sterilized medium. Add 0.5ml of medium to the stem cells of the archaea Aeropyrum pernix K1, dissolve and transfer to a test tube containing 5mL of the above medium, mix well; take 0.5ml from it and transfer to a test tube containing 5ml of fresh medium, and culture at 90°C 7 days. Then transfer to Erlenmeyer flask with 100ml of culture medium and shake culture at 90°C for 3 days, and store the cells at -40°C for future use.
[0038] Take 1.5ml of archaeal culture to collect t...
Embodiment 2
[0051] Embodiment 2: recombinant thermophilic esterase / phospholipase A 2 characteristics
[0052] (1) Optimum reaction temperature
[0053] Reaction temperature is an important factor affecting the catalytic activity of enzymes. Generally, thermophilic esterase / phospholipase A 2 The reactivity at high temperature is much higher than at low temperature. Considering the stability of the substrate, the maximum temperature of the enzyme catalytic activity detection here is only 100°C.
[0054] With NBD-PC as the reaction substrate, phospholipase A was determined in the temperature range of 50-100°C 2 vitality. It can be seen from Figure 4(a) that its activity increases with the increase of temperature in the temperature range of 50-90°C, and decreases above 90°C, and the optimum temperature is 90°C.
[0055] Using p-nitrophenol propionate as the substrate, we also studied the esterase activity of the recombinant enzyme at different temperatures. The results are shown in Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com