Rosinyl diterpene modified alpha - phosphoramidate, preparation method, and application for anti tumors
A technology of rosin-based tricyclic diterpene and amino phosphonate, which is applied in the fields of antineoplastic drugs, chemical instruments and methods, and pharmaceutical formulations to achieve high antitumor activity, low toxicity, and improved fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
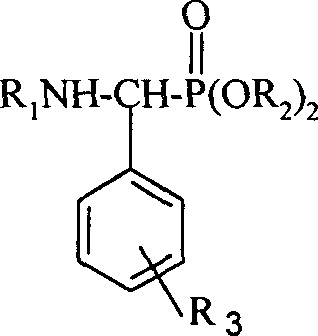
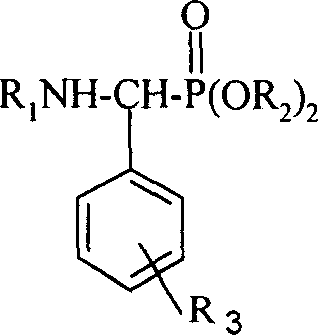
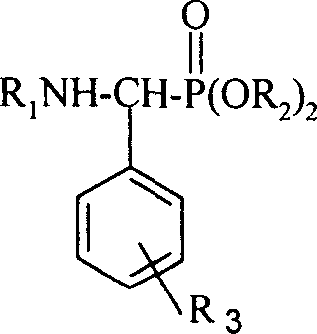
[0027] A kind of α-aminophosphonate modified by rosin-based tricyclic diterpene, its general structural formula is as follows:
[0028]
[0029] Among them, R 1 One of the following substituents:
[0030]
[0031] Dehydroabietyl, abietyl, dihydroabietyl, tetrahydroabietyl,
[0032]
[0033] Degrade dehydroabietyl, degrade abietyl, degrade dihydroabietyl, degrade tetrahydroabietyl,
[0034] R 2 is one of the following groups:
[0035] Me (methyl), Et (ethyl), n-Pr (n-propyl), i-Pr (isopropyl), Bu (butyl), Ph (phenyl);
[0036] R 3 The substitution position is the meta, right, ortho position of the corresponding position on the benzene ring of the externally connected long-chain group, R 3 The represented substituent is one of the following groups or group combinations,
[0037] i.e. R 3 Can be one of the following groups:
[0038] H (hydrogen), o-F (o-fluorine), m-F (m-fluorine), p-F (p-fluorine), p-Cl (p-chlorine), p-CF 3 (p-trifluoromethyl), p-OMe (p-methox...
Embodiment 2
[0042]The preparation method of the α-amino phosphonate of above-mentioned abietyl tricyclic diterpene modification is, with abietyl diterpene amine (as dehydroabietyl, aietyl, dihydroabietyl, tetrahydroabietyl, degradation dehydrogenation Abietyl, degraded aietyl, degraded dihydroabietyl, degraded tetrahydroabietyl), substituted benzaldehyde and phosphite are raw materials to synthesize rosin-based tricyclic diterpene-modified α-aminophosphonate, and the synthesis method is solvent Synthesis method or one-pot synthesis method or solvent-free synthesis method, wherein the ratio of the amount of rosin-based diterpene amine to substituted benzaldehyde and phosphite is 1: (1-1.05): (1-1.1), such as Selected as: 1:1.01:1, 1:1.02:1, 1:1.03:1, 1:1.04:1, 1:1.05:1, 1:1.01:1.01, 1:1.01:1.02, 1:1.01 : 1.03, 1: 1.01: 1.04, 1: 1.01: 1.05, 1: 1.01: 1.06, 1: 1.01: 1.07, 1: 1.01: 1.09, 1: 1.02: 1.01, 1: 1.02: 1.03, 1: 1.02: 1.05 , 1:1.02:1.06, 1:1.02:1.06, 1:1.02:1.09, 1:1.02:1.1, 1:1.03:1....
Embodiment 3
[0053] An abietyl tricyclic diterpene-modified α-aminophosphonate for anti-tumor application, which uses the above-mentioned rosin-based tricyclic diterpene-modified α-aminophosphonate as an active ingredient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com