Method for removing sulfur-containing compound from carbon fuel by catalytic oxidation
A technology for catalytic oxidation and hydrocarbon fuel, applied in the petrochemical field, can solve the problems of high price of oxidant, difficult extraction and separation, high cost, and achieve the effect of continuous operation, obvious desulfurization effect and overcoming high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
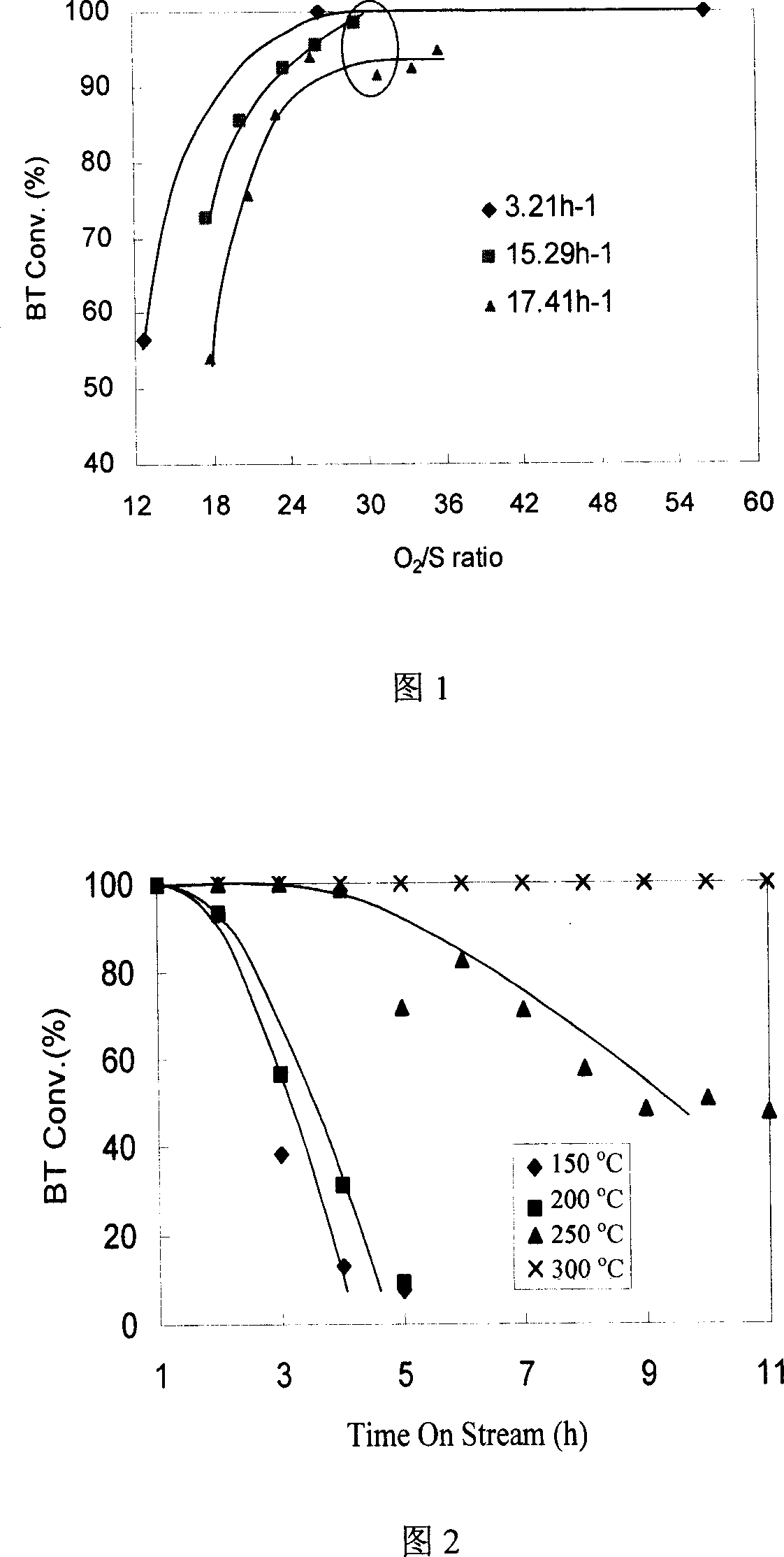
[0021] With 1.5wt%Pd / CeO 2 As a catalyst [synthesis details: Applied Catalysis B, 53 (2004) 77-85], isooctane containing 1000 μg / g sulfur (benzothiophene is the sulfur source) The hourly space velocity is 3.21, 15.29 and 17.41h respectively -1 Under the condition, the O 2 / S ratio, the results are shown in Figure 1. From the results in Figure 1, it can be seen that the best O on the catalyst to achieve the maximum desulfurization rate 2 / S ratio is about 30; at 3.21, 15.29 and 17.41h -1 Under the heavy hourly space velocity, the desulfurization rates are >99%, 98% and 95%, respectively. After identification by PFPD chromatography and mass spectrometer, the sulfur-containing gas in the reaction tail gas is SO 2 . o 2 The selectivity is above 30%.
[0022] 1.5wt%Pt / CeO 2 The same results as in Examples 1-3 were also obtained on the catalyst.
Embodiment 4-6
[0024] 1.5wt%Pt / CeO 2 As a catalyst [synthesis see: Journal of Catalysis, 229 (2005) 499-512], isooctane containing 1000 μg / g sulfur (benzothiophene as sulfur source) is used as raw material, under normal pressure, O 2 / S ratio 30, raw material oil heavy hourly space velocity 15.29h -1 Under the same conditions, the change of benzothiophene removal rate with time at 150, 200, 250, and 300°C was investigated, and the results are shown in Figure 2. It can be seen from the results in Figure 2 that the optimum reaction temperature on this catalyst is ~300 °C; at lower reaction temperatures, the catalyst will be deactivated rapidly.
Embodiment 7-8
[0026] 5wt%Cu / CeO 2 As a catalyst [synthesis details see: Applied Catalysis B, 28 (2000) 113-125], isooctane containing 1000 μg / g sulfur (benzothiophene as sulfur source) is used as raw material, under normal pressure, O 2 / S ratio 17, raw material oil heavy hourly space velocity 40h -1 Under the conditions, the removal rate of sulfur reaches >99% at 300°C, O 2 The selectivity of sulfur is 61.7%; the removal rate of sulfur reaches 97% at 280 ° C, O 2 The selectivity was 59.9%. At normal pressure, O 2 / S ratio 17, raw material oil weight hourly space velocity 80h -1 Under these conditions, the removal rate of sulfur reaches ~70% at 300°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com