Asarone submicron emulsion and its preparation method
A technology of octina submicroemulsion and asarone, which is applied in the direction of antiviral agents, antibacterial drugs, and pharmaceutical formulations, can solve unsatisfactory results and cannot be directly made into nasal sprays or lung inhalants, nasal mucosa or problems such as lung irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
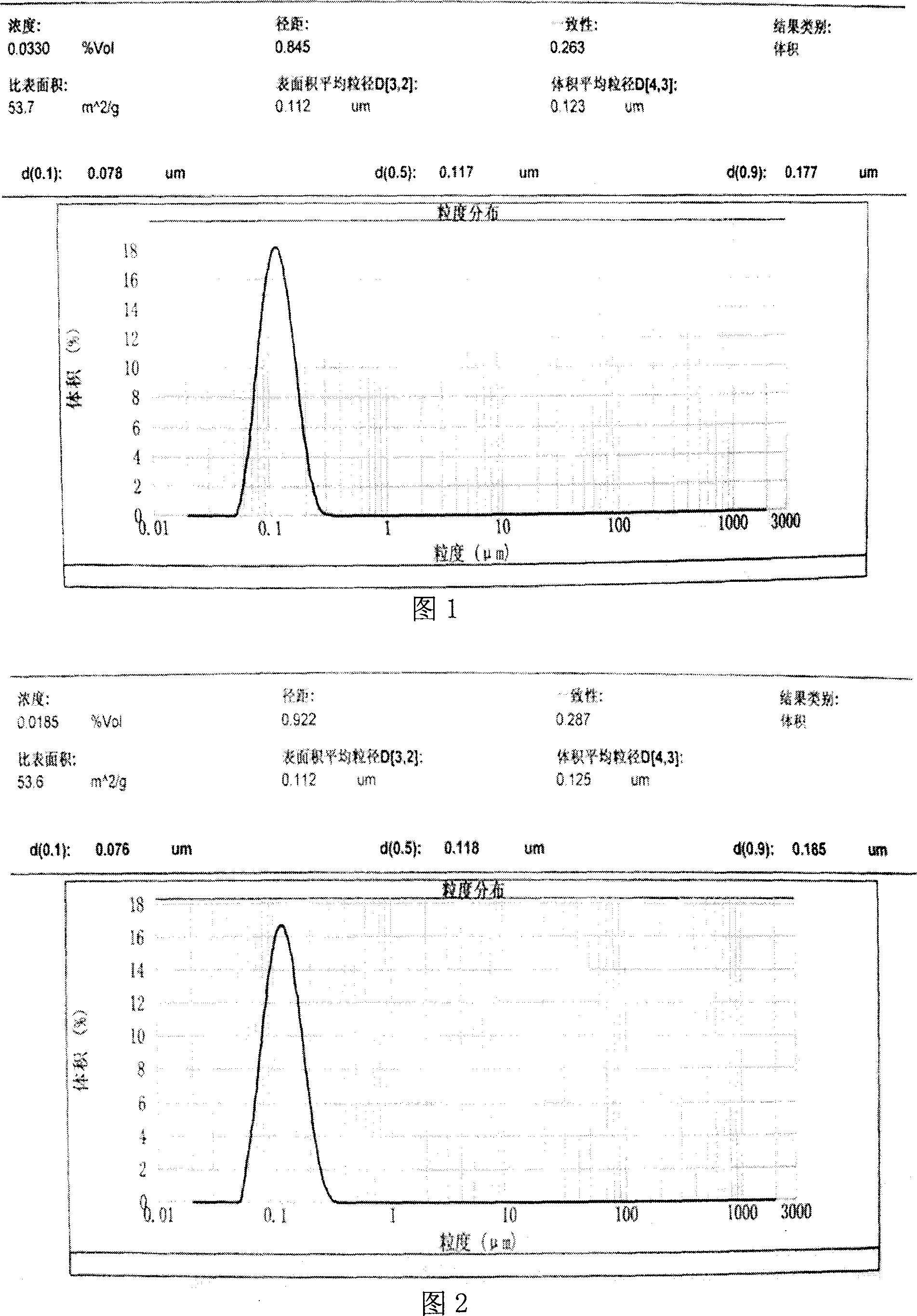
[0040] Asarone feeds at 40mg / ml
[0041] Soybean Oil 20% (ml / ml)
[0042] Lecithin 1.5% (g / ml)
[0043] Poloxamer 188 3% (g / ml)
[0044] Glycerin 2.5% (g / ml)
[0045] Oleic acid 0.6% (g / ml)
[0046] α-tocopherol (antioxidant) 0.05% (g / ml)
[0047] Water for injection 77% (ml / ml)
[0048] Take glycerin and water for injection, heat it to 40-60°C, add refined lecithin and poloxamer, put it in a tissue masher, stir at a high speed to form a uniform water phase, and keep it warm; take asarone and α-tocopherol, add Put it into soybean oil and heat it to 40-60°C to make an oil phase. Slowly add the water phase to the oil phase under the stirring condition, put it in a tissue masher and stir at a high speed to form uniform colostrum. Quickly transfer the colostrum to a high-pressure homogenizer for homogenization, control the particle size below 500nm, collect all the emulsion, adjust the pH of the emulsion to 7.0±0.5 with NaOH, and obtain the product.
Embodiment 2
[0050] [Prescription Composition]
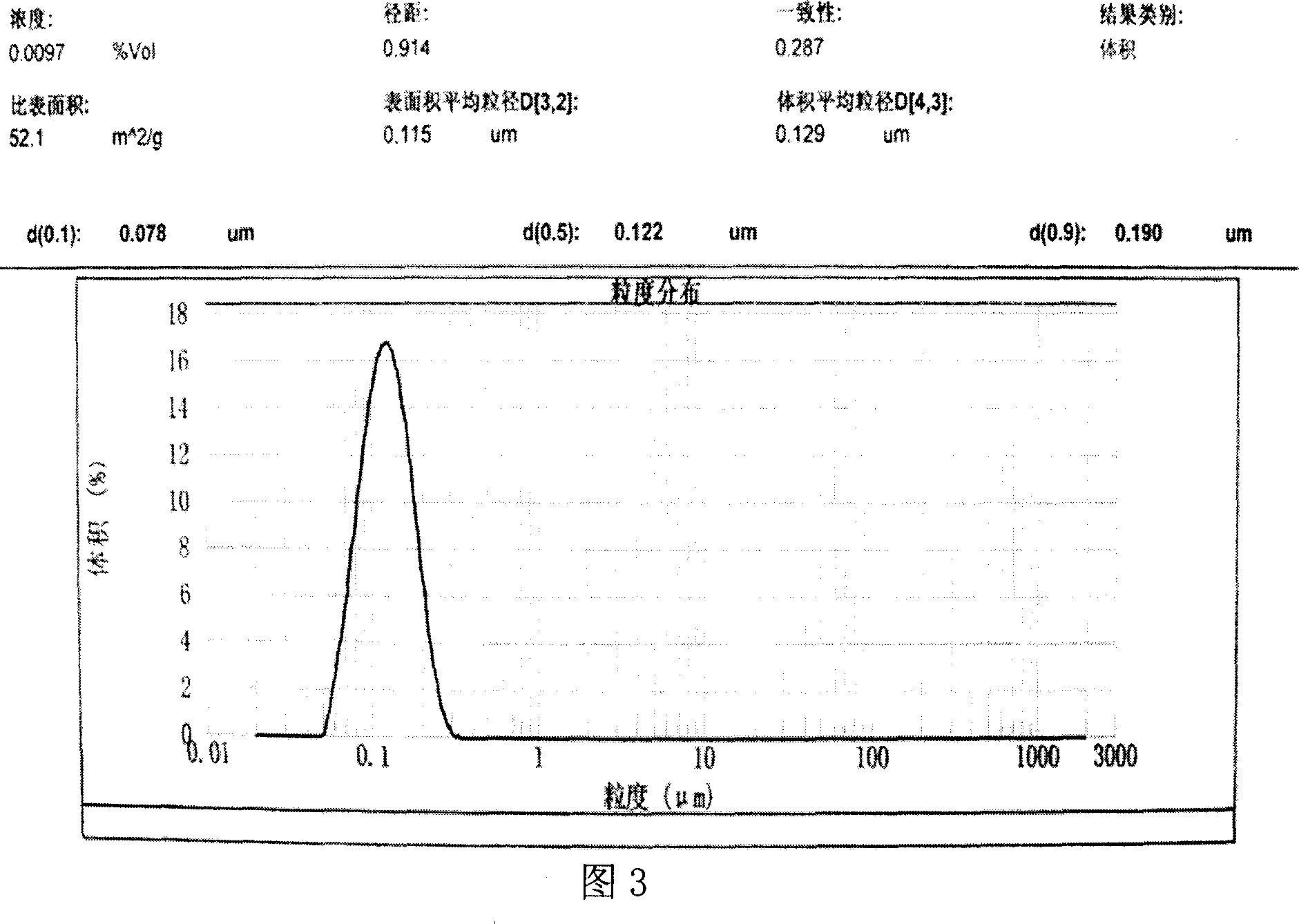
[0051] Asarone feeds at 60mg / ml
[0052] Soybean Oil 25% (ml / ml)
[0053] Lecithin 1.5% (g / ml)
[0054] Poloxamer 188 3% (g / ml)
[0055] Glycerin 2.5% (g / ml)
[0056] Oleic acid 0.6% (g / ml)
[0057] α-tocopherol (antioxidant) 0.05% (g / ml)
[0058] Water for injection about 75% (ml / ml)
[0059] [Preparation method]: with embodiment 1.
Embodiment 3
[0061] [Prescription Composition]
[0062] Asarone fed at 30mg / ml
[0063] Soybean Oil 20% (ml / ml)
[0064] Lecithin 1.5% (g / ml)
[0065] Poloxamer 188 4% (g / ml)
[0066] Mannitol 6.0%
[0067] Oleic acid 0.6% (g / ml)
[0068] α-tocopherol (antioxidant) 0.05% (g / ml)
[0069] Water for injection about 80% (ml / ml)
[0070] [Preparation method]: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com