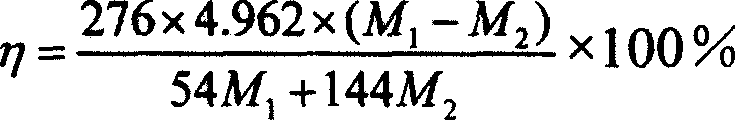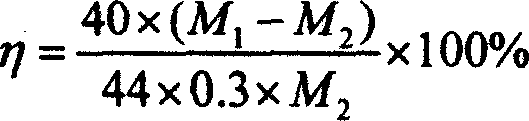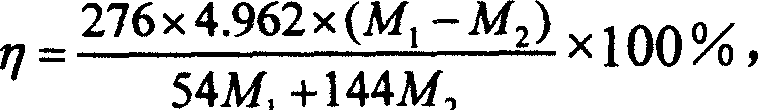CO2 mineralizing process
A carbon dioxide, mineralization technology, applied in the preparation of carbonate/acid carbonate, magnesium carbonate, calcium carbonate/strontium/barium, etc., can solve the problem that high-purity carbon dioxide has not been effectively solved, and achieve reserves Abundant, easy to mine, no environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] Example 1. 1.5 grams of serpentine with 15% by mass of magnesium was crushed and ground to a powder of 200 mesh, and the serpentine ore sample was roasted at a constant temperature of 650°C for 1 hour, and then the roasted ore sample and the catalytic solution (NaHCO 3 :2.016g, NaCl: 2.19g, distilled water: 13.5ml) After mixing evenly, place it in the reactor of the high-pressure reaction system, fill with carbon dioxide, the reaction temperature is 155℃, the reaction pressure is 10MPa; keep the temperature and pressure unchanged and react 60 After 5 minutes, cool down. When the temperature of the system is lower than 85℃, vent the air. Take out the residue in the reactor and dry it at 105℃ for 1 hour. Then take out 1.026 g of the dried residue and send it to the muffle furnace for calcination for 2 hours. , The calcination temperature is 650℃. The calcined product is weighed, and the weighed mass is 0.941 g, which passes the mass M before calcining 1(1.026 g) and the mass ...
example 2
[0031] Example 2. Crushing 2.0 grams of serpentine with 20% by mass of magnesium to a powder of 300 mesh, and roasting the serpentine ore sample at a constant temperature of 650°C for 1 hour, and then the roasted ore sample and the catalytic solution (NaHCO 3 :3.024g, NaCl: 3.216g, distilled water: 16.5ml) After mixing uniformly, place it in the reactor of the high-pressure reaction system, and fill with carbon dioxide. The reaction temperature is 120℃, and the reaction pressure is 12MPa; keep the temperature and pressure unchanged and react 60 Minutes later, cool down. When the temperature of the system is lower than 85°C, let the gas escape. Take out the residue in the reactor and dry it at 105°C for 1 hour. Then take out 1.055 g of the dried residue and send it to the muffle furnace for calcination for 2 hours. , The calcination temperature is 650℃. Weigh the calcined product, the mass after weighing is 0.972 g, which passes the mass M before calcining 1 (1.055g) and the mass a...
example 3
[0032] Example 3. Grind 10 grams of wollastonite with 35% calcium by mass to a powder of 400 mesh, and roast the wollastonite sample at a constant temperature of 650°C for 2 hours, and add 40 ml once to a reactor with a volume of 0.25L Distilled water and 10.0 grams of 400 mesh wollastonite powder were filled with carbon dioxide, the reaction temperature was 150°C, and the reaction pressure was 6MPa; after keeping the temperature and pressure unchanged, after reacting for 60 minutes, cool to room temperature and vent the air. Collect the residue in a beaker. After the upper solution in the beaker is clear, the solution is poured out, and the solid is transferred to a porcelain boat, and dried in a drying oven at 105°C for 24 hours. After the solid product was dried in a drying oven, 1.007 grams were taken out and subjected to a calcination decomposition test at a high temperature, the calcination time was 1 hour, and the calcination temperature was 700°C. Weigh the calcined produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com