Ionic liquid catalyzed transalkylation process for preparing 2,6-dimethyl naphthalene
A technology of ionic liquid and dimethylnaphthalene, which is applied in the field of catalytic transfer alkylation of ionic liquid to prepare 2, can solve the problem of intensified dealkylation, isomerization and polyalkylation, difficult to significantly improve, and separation difficulties, etc. problems, to achieve the effect of no environmental pollution, good effect and wide adaptability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
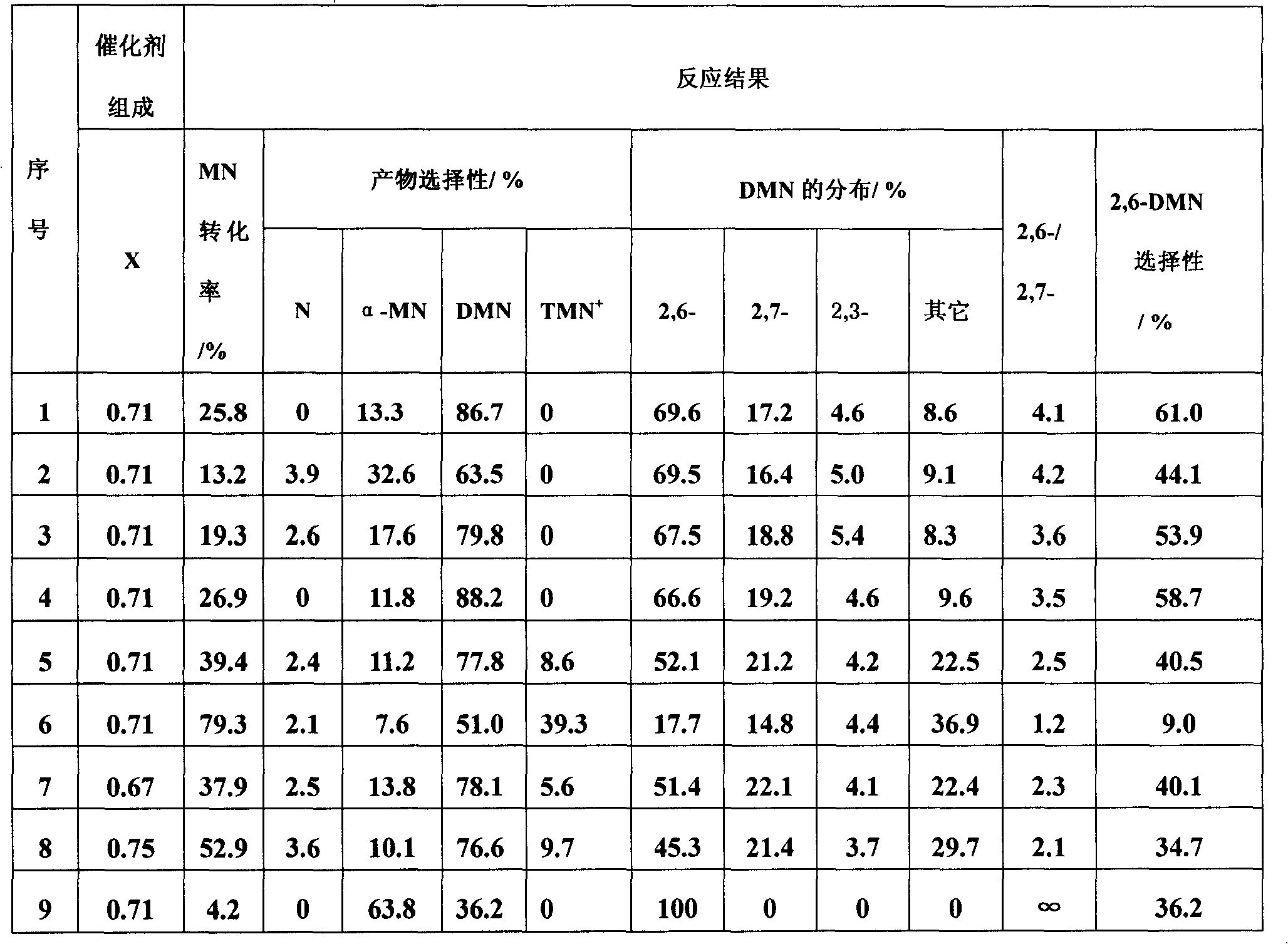
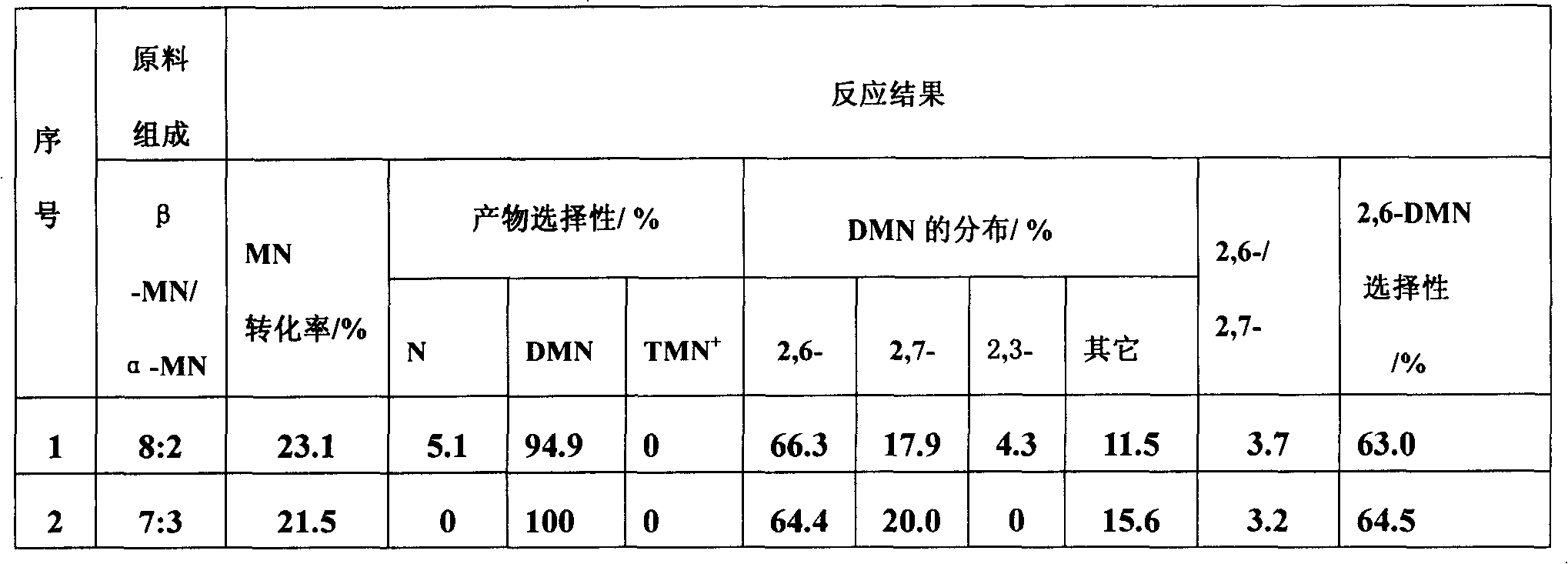
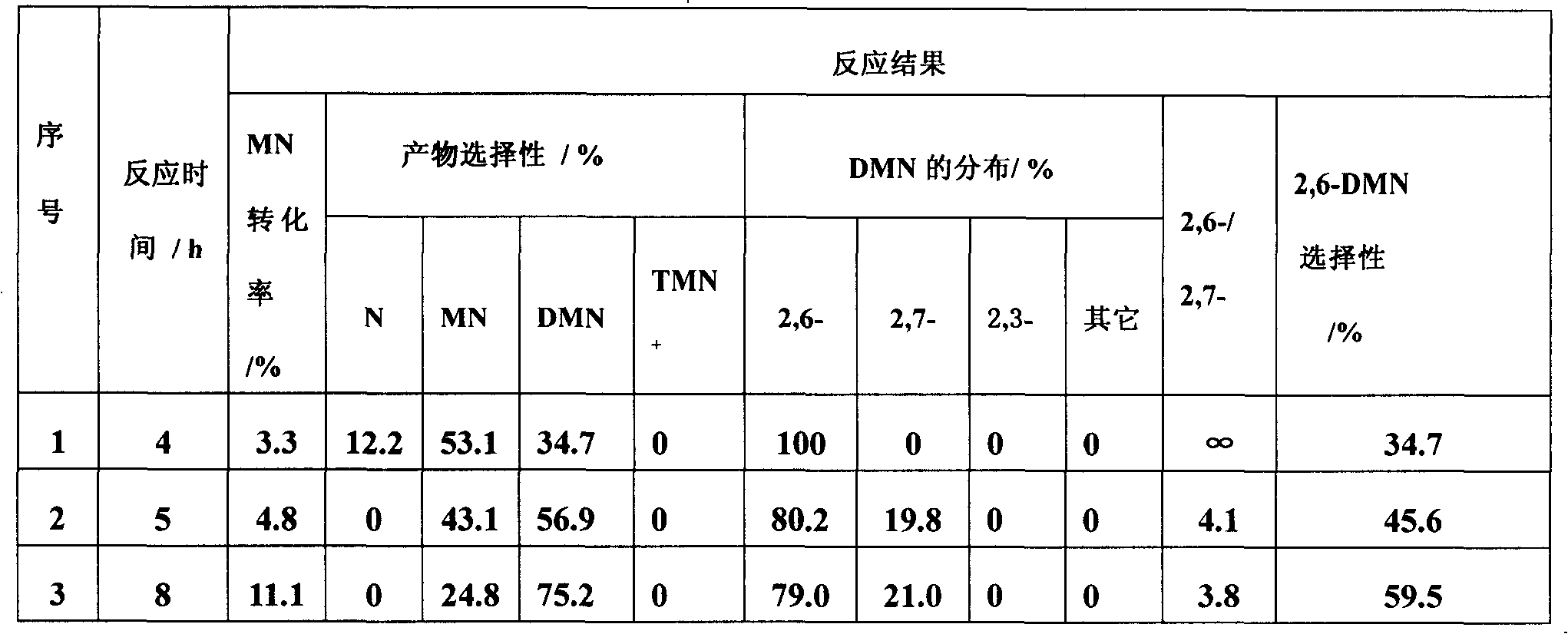
[0026] A method for preparing 2,6-dimethylnaphthalene by catalyzed transfer alkylation of ionic liquid, with β-methylnaphthalene, 1,2,4,5-tetramethylbenzene and cyclohexane in a molar ratio of 1:1:12 Mix, add 47.8% of the ionic liquid catalyst accounting for the total weight of the mixed solution to the mixed solution, and carry out the transfer alkylation reaction at a temperature of 20 ° C under the protection of an inert gas. After 7 hours, the reaction ends, and the natural layering occurs after cooling to room temperature. The liquid is in the lower layer, and the upper layer reaction liquid is decanted to obtain 2,6-dimethylnaphthalene. The remaining ionic liquid can be reused, and the supernatant liquid is taken for qualitative and quantitative analysis of gas chromatography, and the conversion rate of β-methylnaphthalene and the selectivity of 2,6-dimethylnaphthalene are calculated by the area normalization method, See Table 1 (No. 1). The ionic catalyst is the combin...
Embodiment 2
[0028] The ionic liquid catalytic transfer alkylation described in embodiment 1 prepares the method for 2,6-dimethylnaphthalene, with β-methylnaphthalene, 1,2,4,5-tetramethylbenzene and cyclohexane with molar ratio 1 : 0.5: 12 mixing, adding 47.8% of the ionic liquid catalyst accounting for the total weight of the mixed solution in the mixed solution, at a temperature of 20 ° C, under the protection of an inert gas to carry out the transfer alkylation reaction, after 7 hours, the reaction is over, cooled to room temperature naturally Layering, the ionic liquid is in the lower layer, and the upper layer of the reaction solution is decanted to obtain 2,6-dimethylnaphthalene. The remaining ionic liquid can be reused, and the supernatant liquid is taken for qualitative and quantitative analysis of gas chromatography, and the conversion rate of β-methylnaphthalene and the selectivity of 2,6-dimethylnaphthalene are calculated by the area normalization method, See Table 1 (sequence n...
Embodiment 3
[0030]The ionic liquid catalytic transfer alkylation described in embodiment 1 prepares the method for 2,6-dimethylnaphthalene, with β-methylnaphthalene, 1,2,4,5-tetramethylbenzene and cyclohexane with molar ratio 1 : 1: 12 mixing, add in the mixed solution and account for the ionic liquid catalyst of 23.9 or 71.7% of the total weight of the mixed solution, at a temperature of 20°C, carry out the transalkylation reaction under the protection of an inert gas, after 7 hours, the reaction ends, and it is cooled to Natural layering at room temperature, the ionic liquid is in the lower layer, and the upper layer of the reaction solution is decanted to obtain 2,6-dimethylnaphthalene. The remaining ionic liquid can be reused, and the supernatant liquid is taken for qualitative and quantitative analysis of gas chromatography, and the conversion rate of β-methylnaphthalene and the selectivity of 2,6-dimethylnaphthalene are calculated by the area normalization method, See Table 1 (seria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com