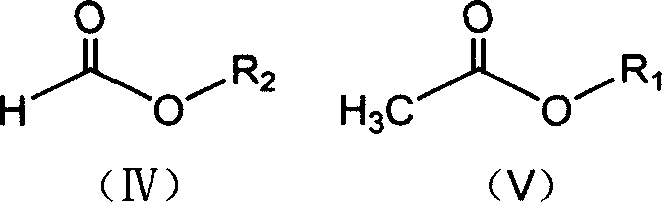Synthesis process of beta-cyclopropylamino acrylate
A technology of cyclopropylamino acrylate and cyclopropylamino acrylic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of high cost, the production of quinolone antibacterial drugs is not widely used, and achieves operation Convenience, reduced emissions, simple response effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
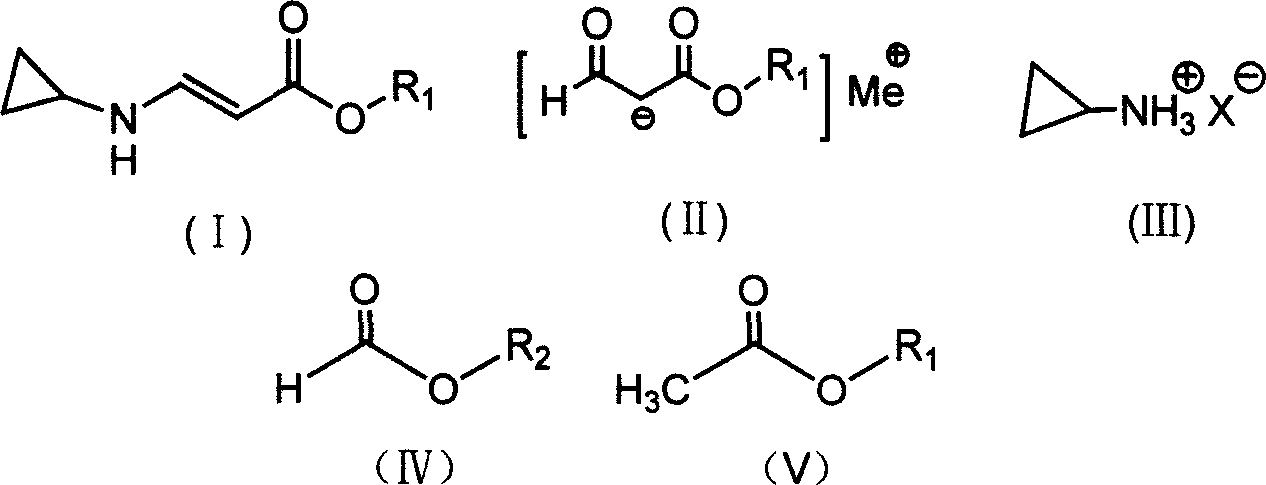
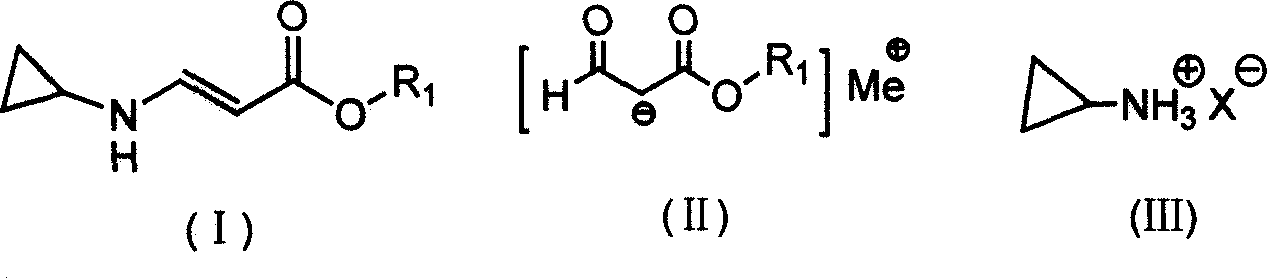
[0041] Press 2.30g of metallic sodium into sodium wire and place it in a reaction bottle, add 50ml of anhydrous ether, control the temperature of the reaction solution at about 25°C, and drop the mixture of 11.8g of ethyl formate and 8.2g of methyl acetate from a constant pressure Slowly add it dropwise in the reaction bottle in the funnel, let it stand for the reaction, the reaction speed is slow at the beginning, when the reaction starts to accelerate after a period of time, a large amount of bubbles are released at the same time, when the metal sodium completely disappears, the reaction ends, and 11.6g formylacetic acid is obtained by filtration Methyl ester sodium salt, yield 93.5%.
Embodiment 2
[0043] Press 2.30g of metallic sodium into sodium wire and place it in a reaction flask, add 45ml of isopropyl ether, mix 11.8g of ethyl formate and 9.7g of ethyl acetate, and slowly drop them into the reaction flask from a constant pressure dropping funnel. In the bottle, control the temperature of the reaction solution at about 30°C. The reaction speed is slow at the beginning. After a period of time, the reaction starts to accelerate, and a large number of bubbles are released at the same time. When the metal sodium completely disappears, the reaction is over, and the sodium formyl acetate is obtained by filtration. Salt 12.7g, yield 92.0%.
Embodiment 3
[0045]Put 15.5g of cyclopropylamine sulfate into a reaction bottle containing 30ml of petroleum ether (60-90°C) (product of Hangzhou Shengli Chemical Co., Ltd.), heat up and stir to reflux, and then add 13.8g of ethyl formyl acetate sodium salt Slowly add the suspension mixed with 50ml of petroleum ether (60-90°C) into the reaction solution dropwise, the dropwise addition is completed in about 1.5 hours, and the reaction is completed after 4.5 hours of reaction, stop the reaction, cool, filter out the solid salt, and the mother liquor is under normal pressure Low boilers and most of the solvents were distilled off, and then distilled under reduced pressure to collect 14.2 g of 64-68°C / 12Pa fraction, namely ethyl β-cyclopropylaminoacrylate, with a yield of 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com