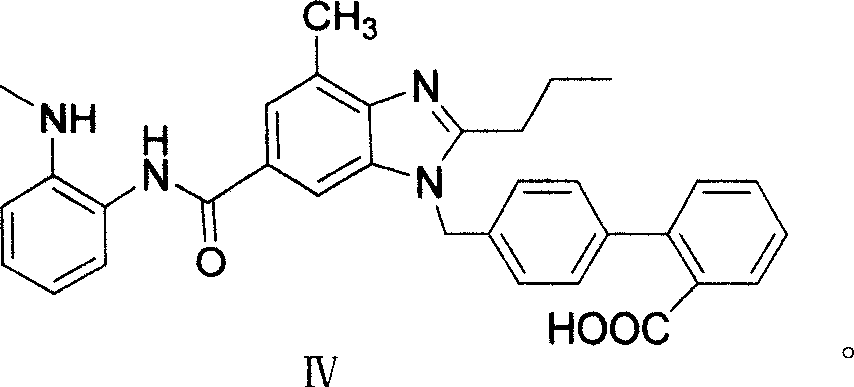
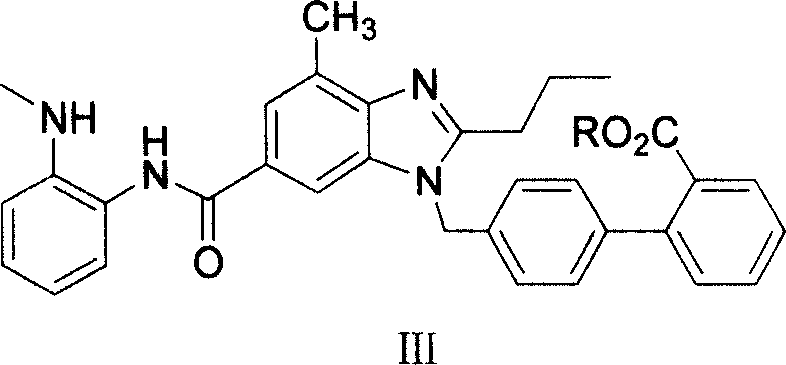
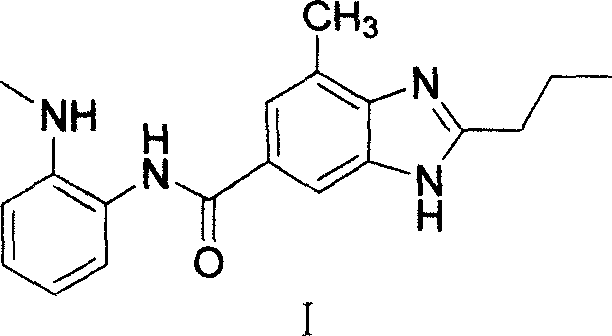
Intermediate of telmisartan, its preparation and use
A technology for telmisartan and an intermediate, which is applied in the field of preparing telmisartan, can solve the problems of short reaction time, little environmental pollution, serious environmental pollution and the like, and achieves the advantages of industrial production, little environmental pollution and safe operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 7-methyl-2-propyl-3H-benzimidazole-5-formyl chloride
[0039] Add 11g of 7-methyl-2-propyl-3H-benzimidazole-5-carboxylic acid, 100ml of thionyl chloride and 1ml of DMF into a 250ml three-necked flask, heat and reflux under stirring for half an hour, and recover the excess chloride under reduced pressure. Add 100ml of toluene to sulfoxide, stir and suction filter until dry to obtain 7-methyl-2-propyl-3H-benzimidazole-5-carbonyl chloride, which is directly used in the next step.
Embodiment 2
[0040] Example 2: Preparation of 7-methyl-N-(2-(methylamino)phenyl)-2-n-propyl-3H-benzimidazole-5-carboxamide
[0041] Add 9.5 g of N-methyl o-phenylenediamine hydrochloride and 300 ml of dichloromethane into a 500 ml three-necked flask, stir and cool to 0-5°C, then add the benzimidazole chloride obtained in the previous step. Add triethylamine dropwise while keeping warm, continue to stir for half an hour after the dropwise addition is completed, filter the reaction solution with suction, concentrate the filtrate to dryness, dissolve the residue in 300ml of 5% dilute hydrochloric acid, and dissolve it with 5% sodium hydroxide solution under stirring. The pH value of the solution was adjusted to 11, a white solid was precipitated, filtered with suction, the filter cake was washed with water to neutrality, and dried under an infrared lamp to constant weight to obtain 7-methyl-N-(2-(methylamino)phenyl)- 2-n-propyl-3H-benzimidazole-5-carboxamide, the yield is 83%.
Embodiment 3
[0042] Example 3: 4'-((4-methyl-6-(2-methylamino)phenylcarbamoyl)-2-n-propyl-1H-benzimidazol-1-yl)methyl)biphenyl - Preparation of methyl 2-carboxylate
[0043] Add 7-methyl-N-(2-(methylamino)phenyl)-2-n-propyl-3H-benzimidazole-5-carboxamide 10g, 300ml acetonitrile in a 500ml three-necked flask protected by nitrogen, Stir and cool to between 0 and 5°C, add 4g of sodium hydride and stir for 10 minutes, add 9g of 4'-bromomethylbiphenyl-2-benzyl carboxylate in batches under heat preservation, continue stirring for half an hour after the addition, the reaction solution Pour into 500ml of water, extract the aqueous phase with dichloromethane, combine the dichloromethane layers, and concentrate to dryness to give 4'-((4-methyl-6-(2-methylamino)phenylcarbamoyl)-2- n-Propyl-1H-benzimidazol-1-yl)methyl)biphenyl-2-carboxylic acid methyl ester. Yield 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com