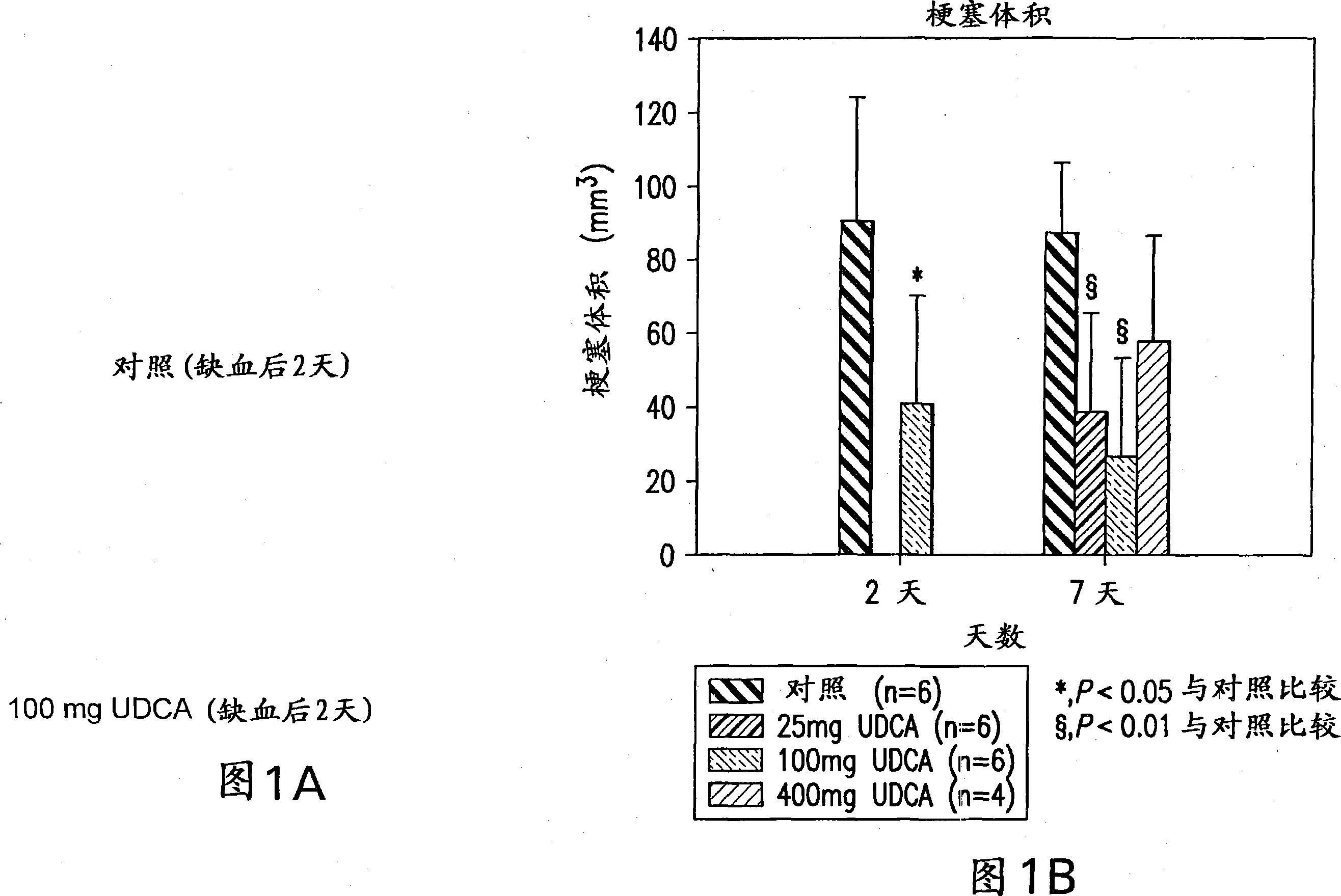
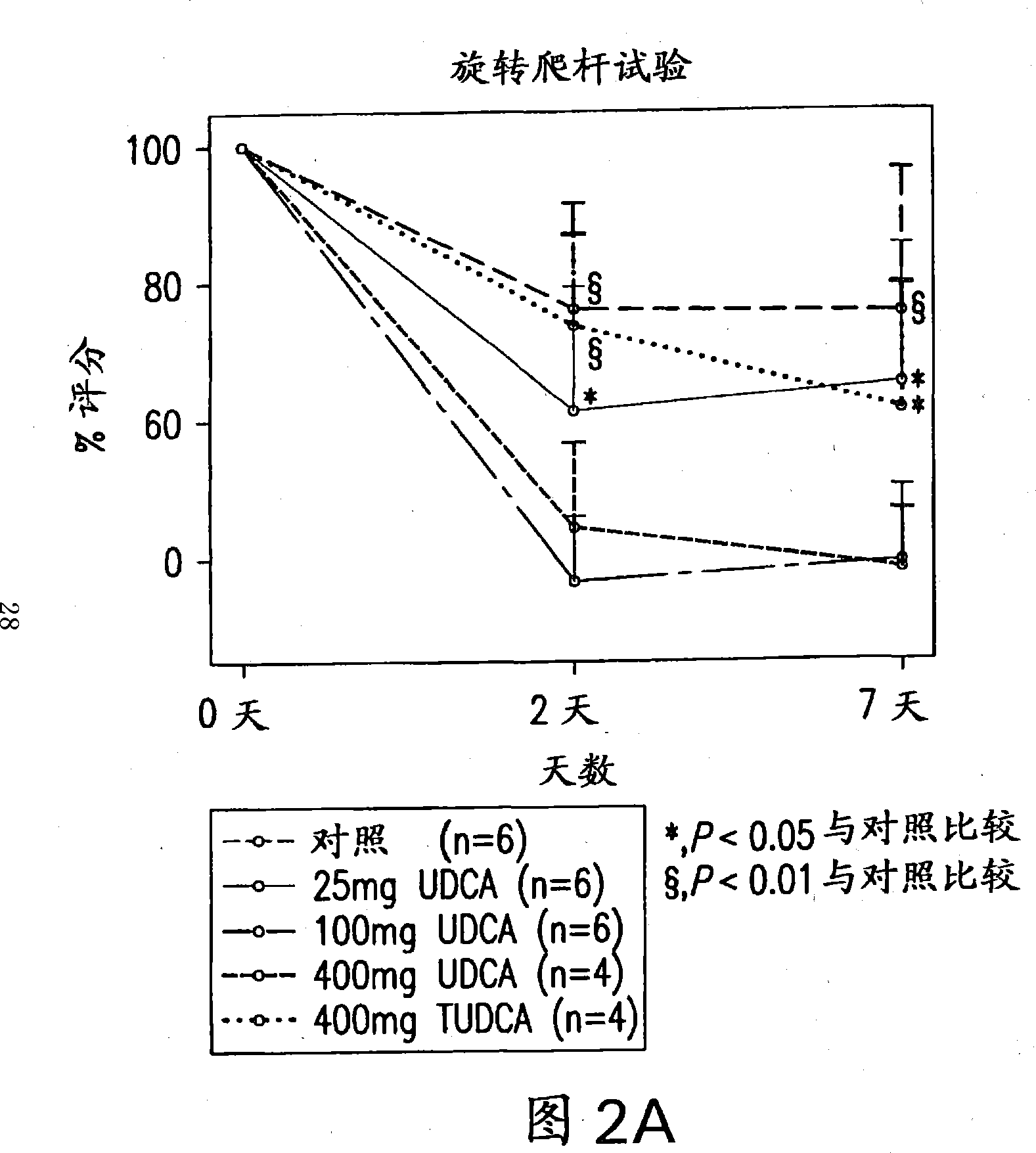
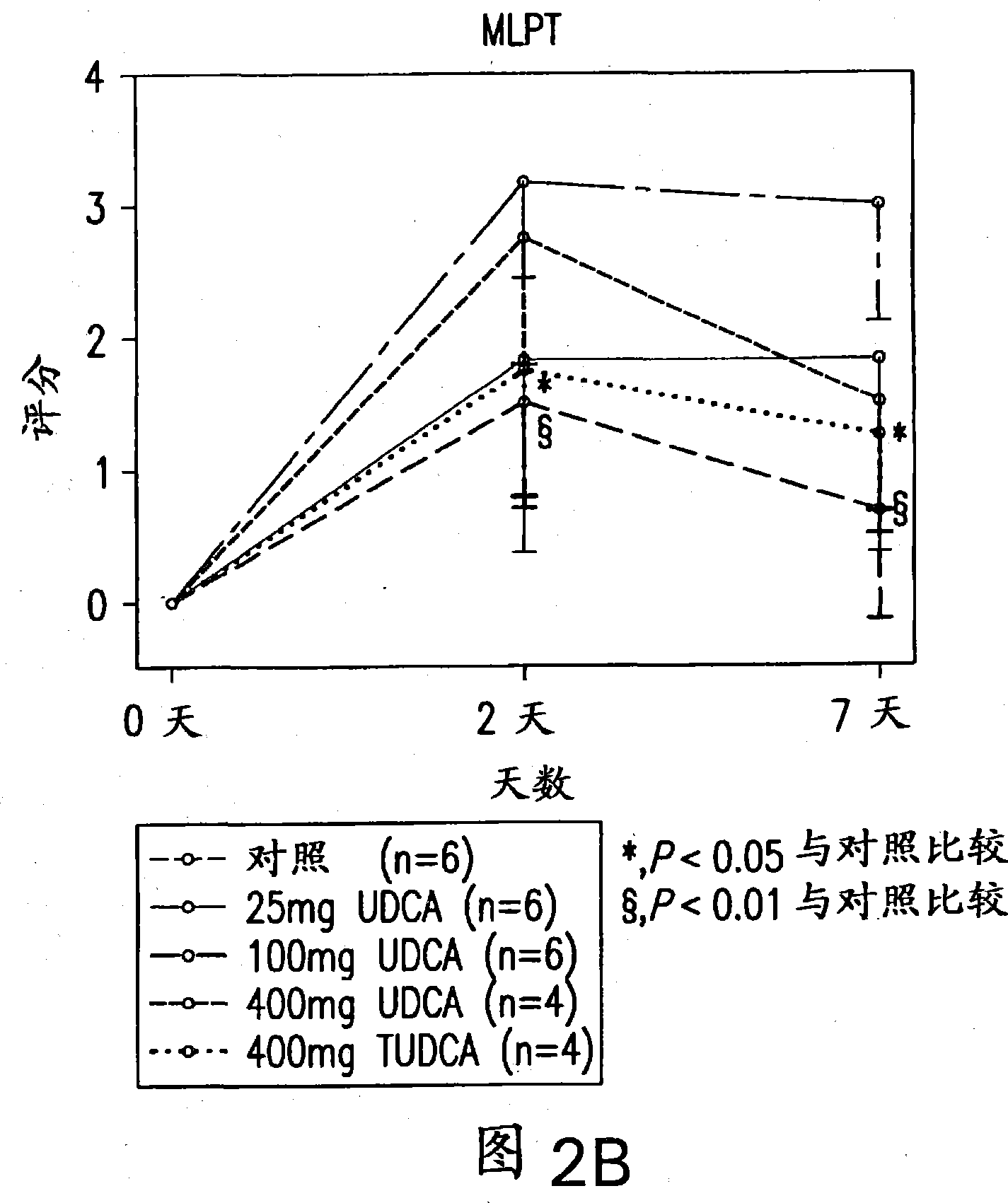
Neuroprotective effect of solubilized UDCA in focal ischemic model
An ischemic stroke and solution technology, which can be used in nervous system diseases, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can solve problems such as nerve damage and lack of therapeutic intervention.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Soluble UDCA Formulation
[0061] A clear aqueous solution of solubilized UDCA (s-UDCA) containing intact UDCA and low glucose titer water-soluble starch was prepared. Briefly, 6.48 g of sodium hydroxide particles were dissolved in 500 ml of purified water, followed by dissolving 60 g of UDCA in this sodium hydroxide solution at room temperature with stirring. Then, 400 g of carbohydrate (maltodextrin) was added little by little to the clear solution and stirred. Sweetening agents, preservatives (and / or sweetening and flavoring agents) are added to the resulting clear solution in amounts suitable for pharmaceutical formulations. The pH of the clear solution was adjusted to 7-7.5 with sodium hydrogen phosphate. Purified water was added to make the total volume 1000 mL. If necessary, the clear solution was filtered through a sterile cup filter unit of 0.22 μm and GP plus membrane.
Embodiment 2
[0062] Example 2: Soluble UDCA Formulation
[0063]A clear aqueous solution of solubilized UDCA (s-UDCA) containing intact UDCA and low glucose titer water-soluble starch was prepared. Briefly, 1.1 g of sodium hydroxide particles were dissolved in 500 ml of purified water, and next, 10 g of UDCA was dissolved in this sodium hydroxide solution at room temperature with stirring. Then, 500 g of carbohydrate (corn syrup solids) was added little by little to the clear solution and stirred. Sweetening agents, preservatives (and / or sweetening and flavoring agents) are added to the resulting clear solution in amounts suitable for pharmaceutical formulations. The pH of the clear solution was adjusted to 7-7.5 with sodium hydrogen phosphate. Purified water was added to make the total volume 1000 mL.
[0064] The Cmax of this composition was 20.4 μg / mL, which was 7 times higher than the reported actigall produced by Novartis Pharmaceuticals, Newark, New Jersey. Data from "Results of ...
Embodiment 3
[0066] Example 3: Soluble UDCA Formulation
[0067] A clear aqueous solution of solubilized UDCA (s-UDCA) containing intact UDCA and low glucose titer water-soluble starch was prepared. Briefly, 2.27 g of sodium hydroxide particles were dissolved in 100 ml of purified water, followed by dissolving 25 g of UDCA in this sodium hydroxide solution at room temperature with stirring. Separately, 745 g of carbohydrate (maltodextrin) was added little by little to 400 mL of purified water and stirred until dissolved. The resulting clear carbohydrate solution and UDCA sodium salt solution were combined and stirred. To the resulting clear solution are added sweeteners, preservatives (and / or sweeteners and flavors) in amounts suitable for pharmaceutical formulation. The pH of the clear solution was adjusted to 6.5-7.5 with sodium hydrogen phosphate. Purified water was added to make the total volume 1000 mL.
[0068] At 90 to 95 °C, vacuum in a rotary evaporator (1.3 × 10 -1 Pa) The r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com