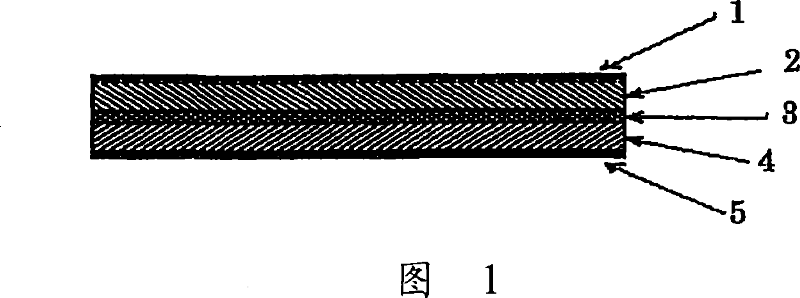
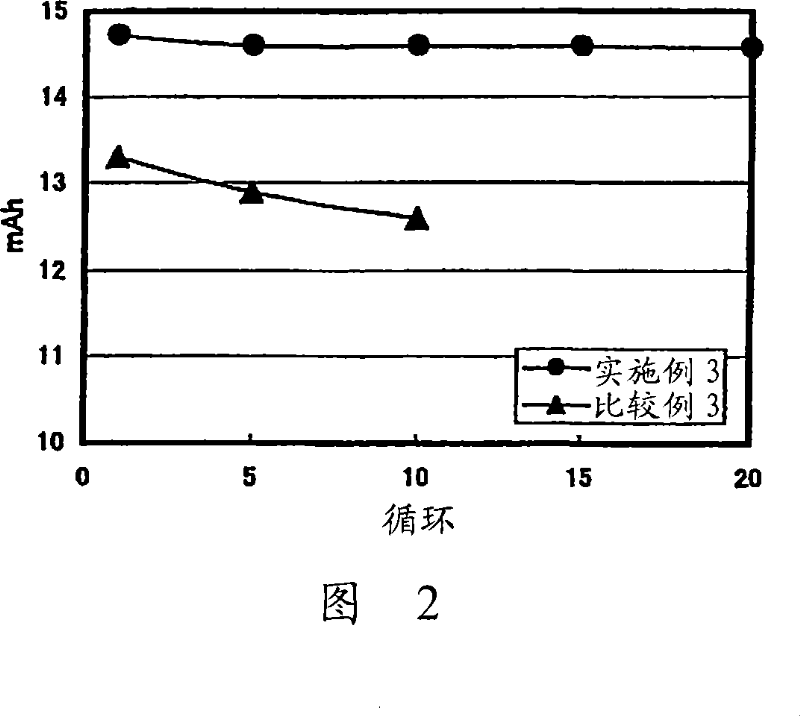
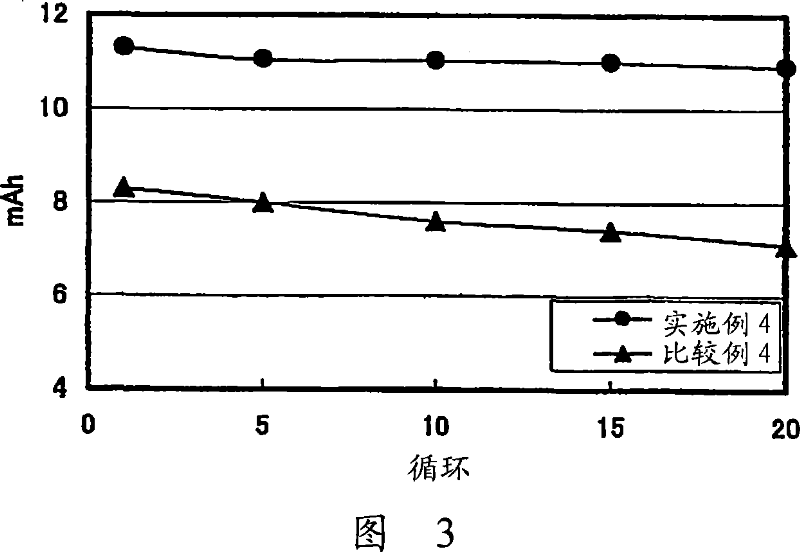
Lithium ion secondary battery and a solid electrolyte thereof
A solid electrolyte, secondary battery technology, applied in secondary batteries, batteries with solid electrolytes, battery electrodes, etc., can solve the problems of impregnation with electrolytic solution and inability to enhance lithium ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Preparation of lithium-ion conductive glass-ceramics:
[0133] Weigh H 3 PO 4 , Al(PO 3 ) 3 , Li 2 CO 3 , SiO 2 and TiO 2 Raw materials and mixed uniformly to make 35.0% P 2 o 5 , 7.5%Al 2 o 3 , 15.0% Li 2 O, 38.0%TiO 2 and 4.5% SiO 2 (expressed as mole percent as oxide). The mixture was placed in a platinum tank and heated and melted in an electric furnace at 1500° C. for three hours while stirring the molten glass. Next, the melt was dropped into running water to produce glass flakes. The glass was heated at 950° C. for twelve hours to crystallize and thereby obtain the target glass-ceramic. By powder X-ray diffraction, it was confirmed that the main crystal phase precipitated was Li 1+x+y Al x Ti 2-x Si y P 3-y o 12 (0≤x≤0.4, 0<y≤0.6). The resulting glass-ceramic flakes were pulverized with a ball mill to obtain glass-ceramic fine particles having an average particle diameter of 2 µm and a maximum particle diameter of 8 µm.
[0134] Preparatio...
Embodiment 2
[0139] Preparation of the positive electrode:
[0140] Using commercially available LiCoO 2 (average particle diameter 6 μm) as the active material of the positive electrode. Combining this positive electrode active material with the addition of acetylene black as an electronically conductive additive and lithium salt LiBF as an ionically conductive additive 4 The copolymer of polyethylene oxide and polypropylene oxide and adhesive are mixed in ethanol solvent. The resulting mixture was uniformly coated on an aluminum plate having a thickness of 16 μm constituting a positive electrode collector and dried at 120° C. to produce a positive electrode in the form of a sheet. The positive electrode had a thickness of 100 μm.
[0141] Preparation of negative electrode:
[0142] Commercially available graphite powder (average particle diameter: 10 μm) was used as the negative electrode. Combining this negative electrode material with the addition of lithium salt LiBF as an ion-co...
Embodiment 3
[0149] Preparation of solid electrolyte:
[0150] The glass-ceramic powder obtained in Example 1 was mixed with polyethylene oxide, polypropylene oxide, and 2-methoxyethoxyethyl shrinkage of LiTFSI (lithium bistrifluoromethanesulfonimide) The copolymer of glyceryl ether was uniformly mixed in ethyl methyl ketone solvent at a ratio of 75:25. The mixture was then coated on a PET film to which release treatment had been applied and dried at room temperature and then further dried at 130° C. under reduced pressure to remove the solvent by evaporation. Another PET film to which peeling treatment had been applied was adhered to the solid electrolyte thus obtained. The composite electrolyte was then heated at 130° C. and pressed with a roll press to remove air bubbles remaining in the composite electrolyte. The PET film on both sides of the solid electrolyte is then peeled off. The obtained solid electrolyte had a thickness of 35 μm.
[0151] Preparation of the positive electrode...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com