Universal synthesizing method for lanthanide series rare earth stannate nano powder
A technology of lanthanide rare earths and nanopowders, which is applied in the direction of rare earth metal compounds, tin compounds, nanotechnology, etc., can solve the problems of large particle size, high energy consumption, and poor product dispersion, so as to reduce energy consumption, simplify the process, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
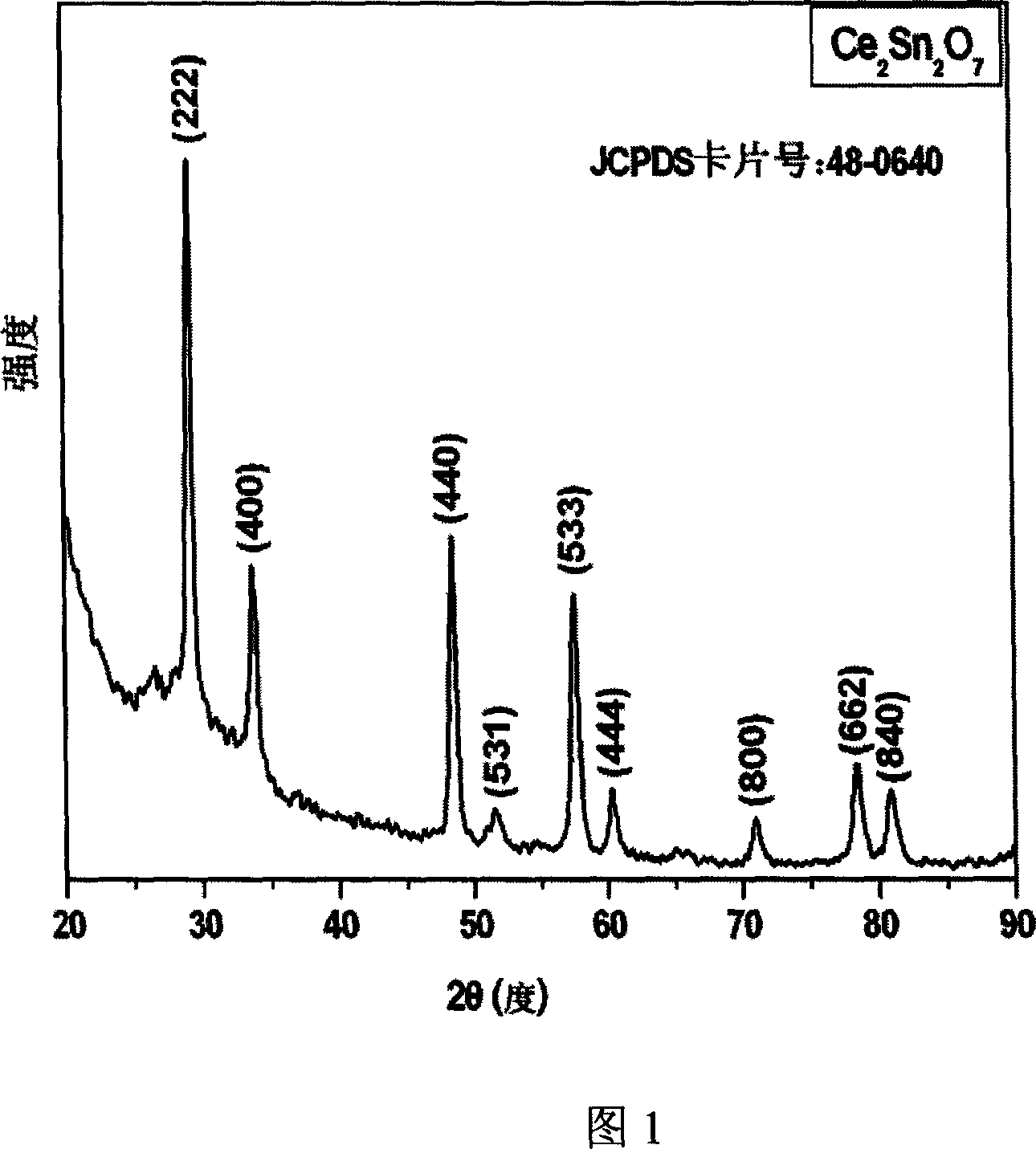
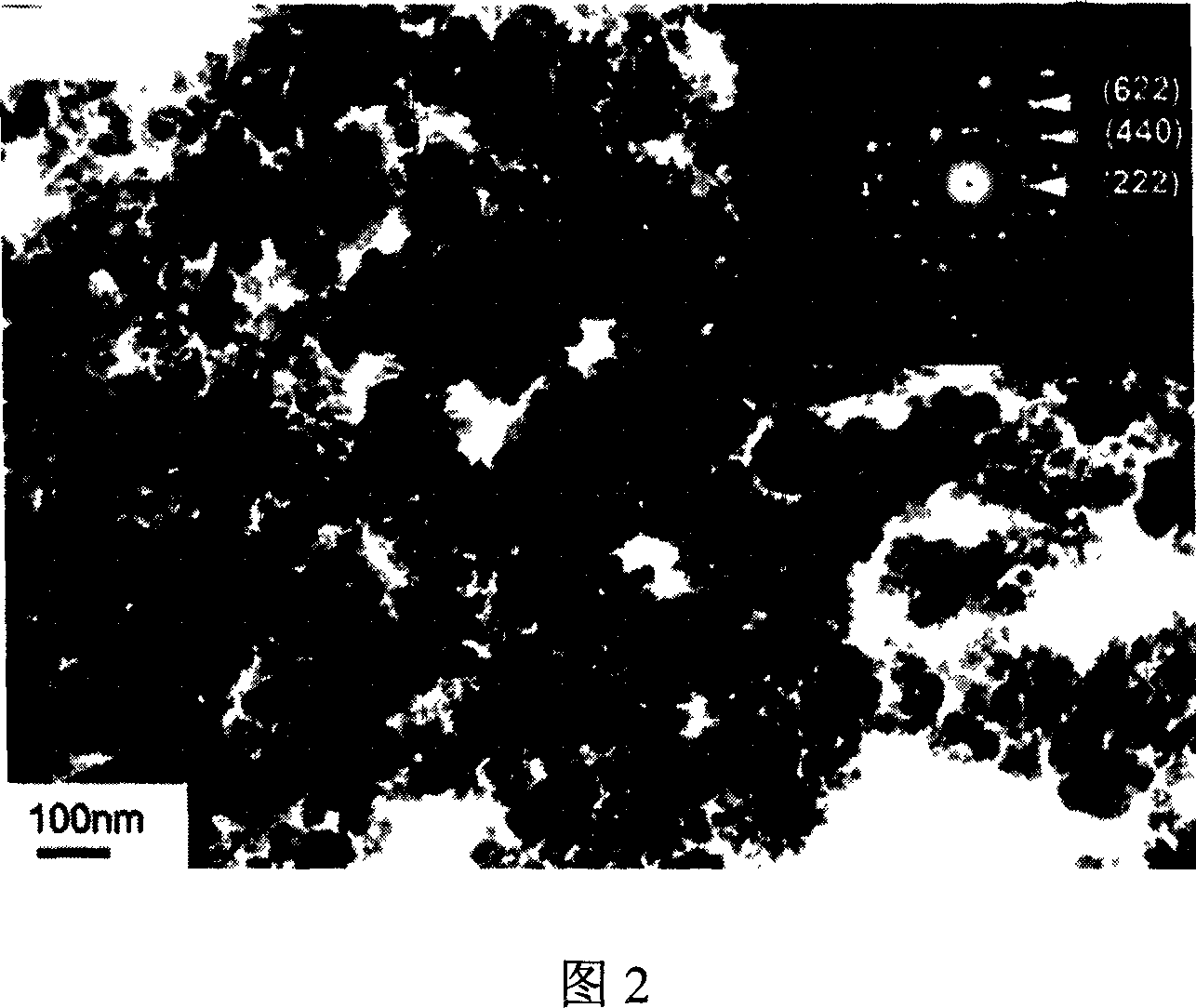
[0029] 0.694 grams of hydrated cerium nitrate (Ce(NO 3 ) 3 ·6H 2 O) be dissolved in 160 milliliters of water, the molar concentration of cerium nitrate is 0.01 mol / liter, stir. Add 0.427 gram of sodium stannate hydrate (Na 2 SnO 3 ·3H 2 (0), the molar concentration of sodium stannate 0.01 mol / liter, fully stirred. The above prepared solution is put into the polytetrafluoroethylene lining of the autoclave, the volume of the lining is 200 milliliters, and the filling degree is controlled to be 80%. The solution was treated at 180°C for 48 hours, and the treated solution was centrifuged and dried to obtain cerium stannate nanopowder with the chemical formula Ce 2 sn 2 o 7 . Figure 1 is the XRD spectrum of the product. All the diffraction peaks in Figure 1 are in perfect agreement with the standard sample card of cerium stannate (JCPDS no.48-0640), indicating that the product is a well-crystallized pyrochlore-type cerium stannate. Fig. 2 is a transmission electron microg...
Embodiment 2
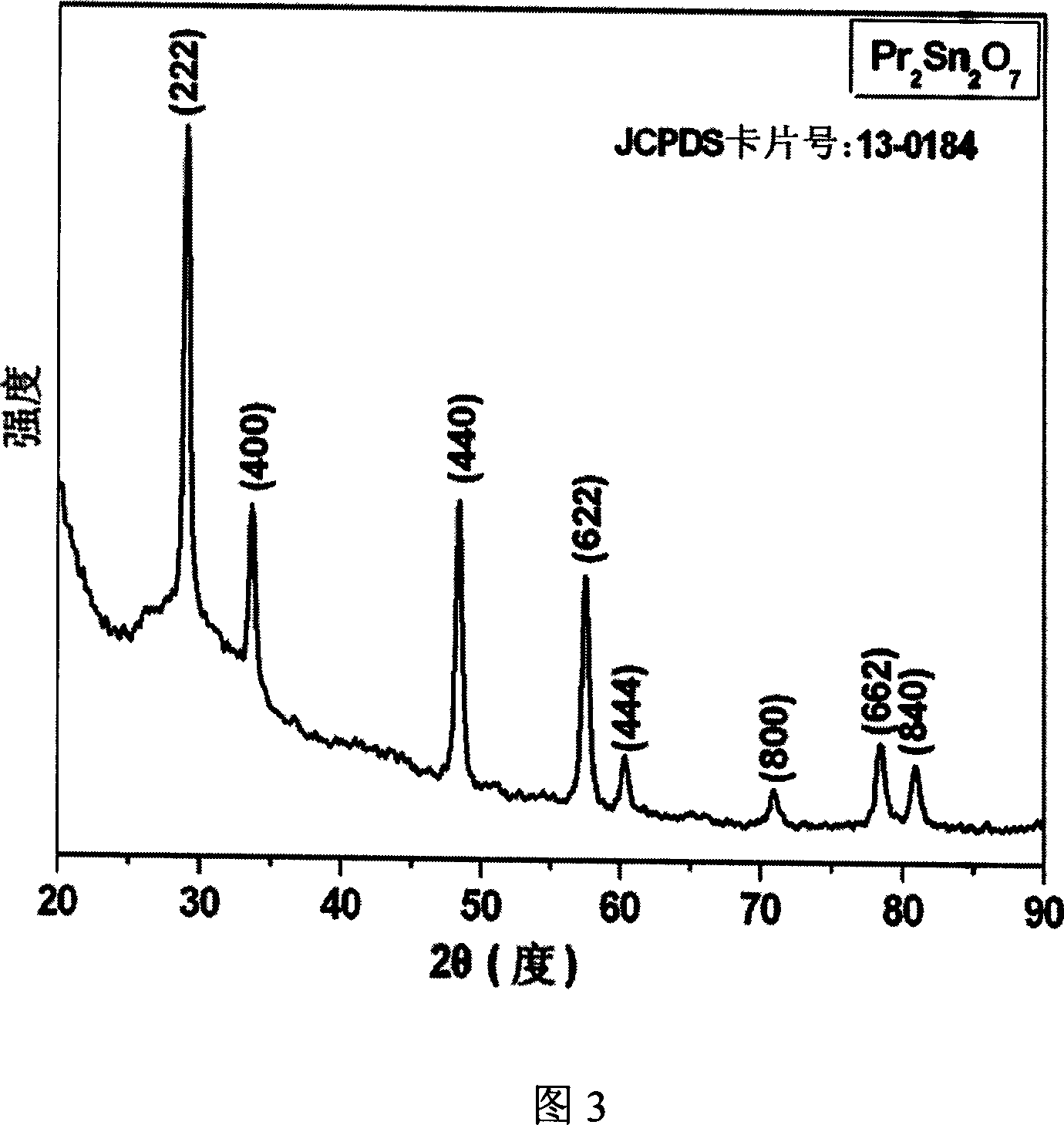
[0031] 2.088 grams of hydrated praseodymium nitrate (Pr(NO 3 ) 3 ·6H 2 O) be dissolved in 160 milliliters of water, the molar concentration of praseodymium nitrate is 0.03 mol / liter, stir. Add 1.281 grams of sodium stannate hydrate (Na 2 SnO 3 ·3H 2(0), the molar concentration of sodium stannate 0.03 mol / liter, fully stirred. Put the above prepared solution into the polytetrafluoroethylene lining of the autoclave, the volume of the lining is 200 milliliters, and the control filling degree is 90%. The solution was treated at 200°C for 100 hours, and the treated solution was centrifuged and dried to obtain praseodymium stannate nanopowder, the chemical formula is Pr 2 sn 2 o 7 . Figure 3 is the XRD pattern of the product. All the diffraction peaks in Figure 3 are in perfect agreement with the standard sample card of praseodymium stannate (JCPDS no.13-0184), indicating that the product is pyrochlore-type praseodymium stannate with good crystallization. Fig. 4 is a trans...
Embodiment 3
[0033] 2.102 grams of hydrated neodymium nitrate (Nd(NO 3 ) 3 ·6H 2 O) be dissolved in 160 milliliters of water, the molar concentration of neodymium nitrate is 0.03 mol / liter, stir. Add 1.281 grams of sodium stannate hydrate (Na 2 SnO 3 ·3H 2 (0), the molar concentration of sodium stannate 0.03 mol / liter, fully stirred. The above prepared solution is put into the polytetrafluoroethylene lining of the autoclave, the volume of the lining is 200 milliliters, and the filling degree is controlled to be 80%. Treat the solution at 200°C for 10 hours, centrifuge and dry the treated solution to obtain neodymium stannate nanopowder, the chemical formula is Nd 2 sn 2 o 7 . Figure 5 is the XRD pattern of the product. All the diffraction peaks in Figure 5 are in perfect agreement with the standard sample card of neodymium stannate (JCPDS no.13-0185), indicating that the product is a pyrochlore-type neodymium stannate with good crystallization. Fig. 6 is a transmission electron m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com