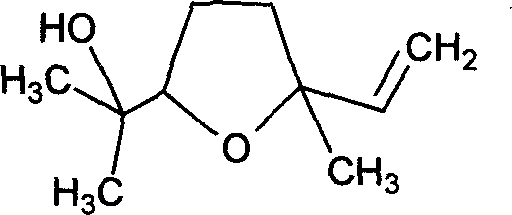
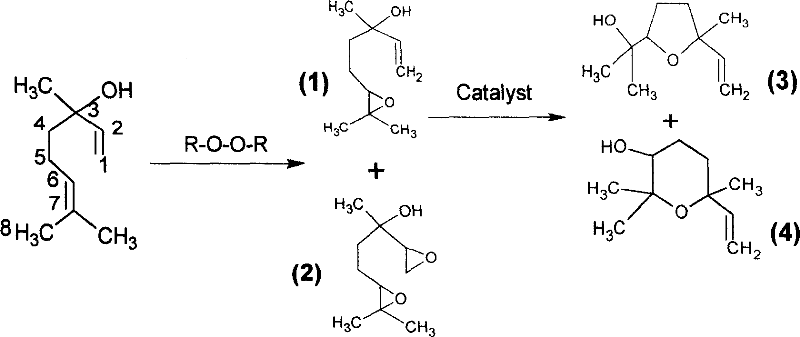
Novel production technique for linalool oxide
A technology for oxidizing linalool and a production process, which is applied in the production process of organic spices and a new production process for oxidizing linalool, can solve problems such as low yield, harsh process conditions, and unstable reaction operation process, and achieve The effect of abundant sources, low price and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 250g of linalool, 600g of sodium peroxyborate (containing effective oxygen ~ 15%) and 1000ml of ethyl acetate into a 2000ml three-necked flask, add 300g of acetic anhydride dropwise with vigorous stirring, and control the reaction temperature at 10°C. After the addition, continue to react and be followed by GC. The conversion of linalool was completed in about 45 hours, the inorganic substances were filtered out, and the reaction mixture was used to remove residual peroxy compounds by conventional methods. Then add 5ml concentrated hydrochloric acid, control the temperature at 30°C and stir for 6 hours, alkali wash, wash with water to neutrality, recover the solvent, vacuum distillation, collect the 72~74°C / 0.8kPa fractions to obtain 175g of linalool oxide. The rate is 70%, the content detected by GC is ≥95%, and the product has a pure aroma.
Embodiment 2
[0026] Add 250g of linalool, 800g of sodium peroxotungstate (containing effective oxygen ~ 8%) and 1000ml of benzene into a 2000ml three-necked flask, add 350g of phthalic anhydride dropwise with vigorous stirring, and control the reaction temperature at 40℃. After that, the reaction was continued and followed by GC. The conversion of linalool was completed in about 26 hours, the inorganic matter was removed by filtration, and the reaction mixture was used to remove residual peroxy compounds by conventional methods. Then add 20g perfluorosulfonic acid resin, control the temperature at 60°C and stir for 4 hours, wash with alkali, wash with water to neutrality, recover the solvent, vacuum distillation, collect 72~74°C / 0.8kPa fractions to obtain linalool oxide 160g, the yield is 64%, and the content detected by GC is ≥95%.
Embodiment 3
[0028] Add 250g of linalool, 400g of sodium peroxycarbonate (containing effective oxygen ~13%) and 1000ml of petroleum ether into a 2000ml three-necked flask, and add 350g of maleic anhydride dropwise with vigorous stirring. The reaction temperature is controlled at 65°C. After finishing, continue to react and be followed by GC. The conversion of linalool was completed in about 16 hours, the inorganic matter was removed by filtration, and the reaction mixture was used to remove residual peroxy compounds by conventional methods. The solvent is recovered, and the fraction at 72~120℃ / 1kPa is collected by vacuum distillation to obtain about 240g crude product. Put the above crude product into a 500ml three-necked flask, add 2g of boron trifluoride-ether, control the temperature at 80℃ and stir for 3 hours, alkali wash, wash with water to neutrality, vacuum distillation, collect 72~74℃ / 0.8kPa 180g of linalool oxide was obtained, the yield was 74%, and the content detected by GC was ≥95...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com