Application of gambogicacid in preparing medication for restraining angiogenesis
A technique for angiogenesis and tumor angiogenesis, which can be used in drug combinations, antipyretics, antitumor drugs, etc., and can solve the problem of lack of gambogic acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
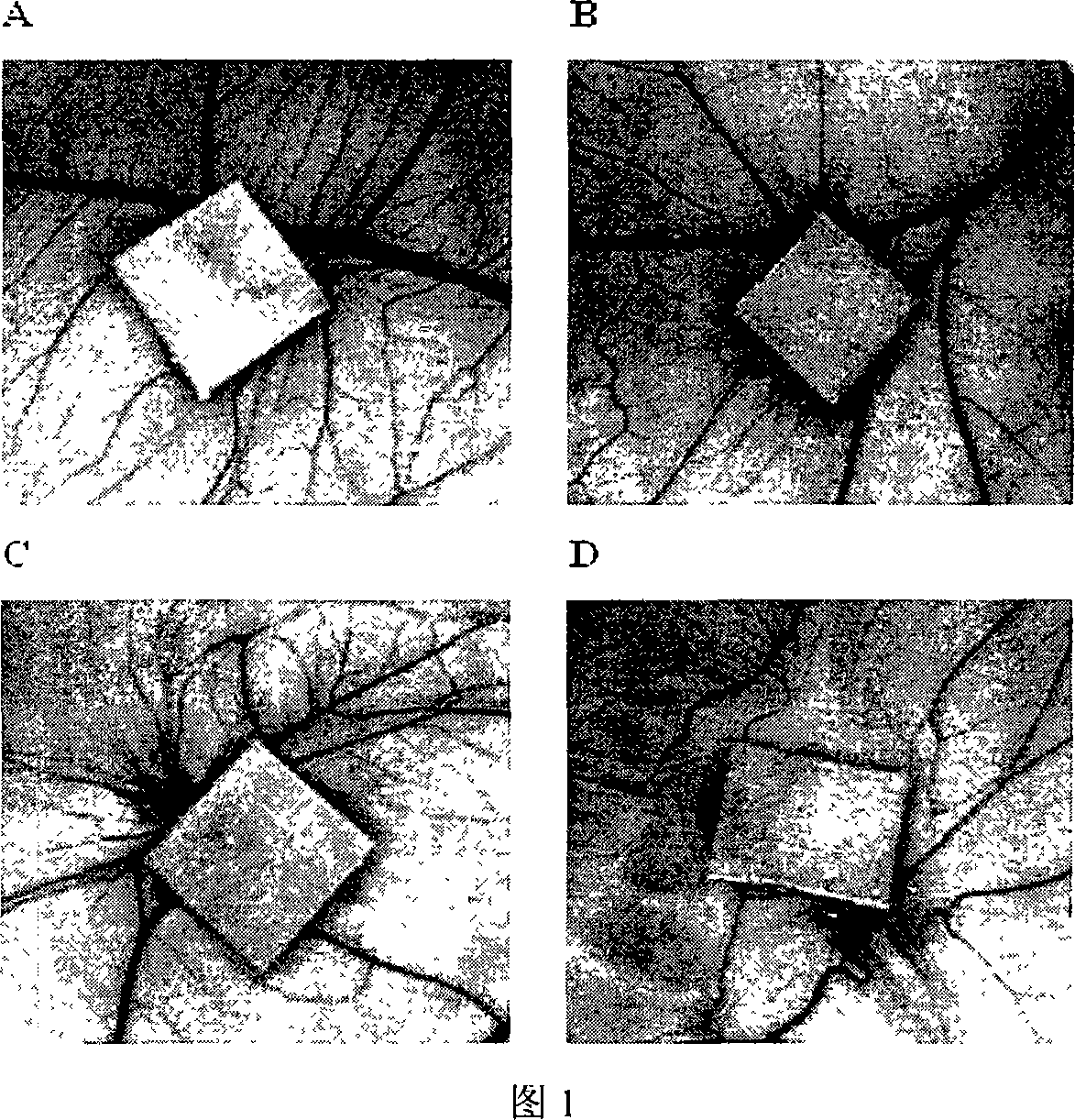
[0020] Example 1: Gambogic acid inhibits angiogenesis of chicken embryo allantoic membrane.
[0021] Purpose and principle: In the early stage of angiogenesis of chicken embryo chorioallantoic membrane, the body’s immune system has not yet been fully established, and there is almost no rejection reaction to various foreign bodies. The drug-containing carrier is placed on its surface, and the effect of the drug on angiogenesis can be observed. influences. Because of its sensitivity to anti-angiogenic drugs, it is still considered an ideal drug screening model.
[0022] Method: The fertilized chicken embryos were placed in the incubator for 9 days (the air cell side is up). Then wipe the chicken embryo with alcohol and let it stand for 15 minutes, draw a 2×2cm window opening position above the chicken embryo, grind and cut the window with scissors, blow off the grinding dust on the eggshell, and remove the window. Then use iris scissors to cut the shell membrane, exposing the ...
Embodiment 2
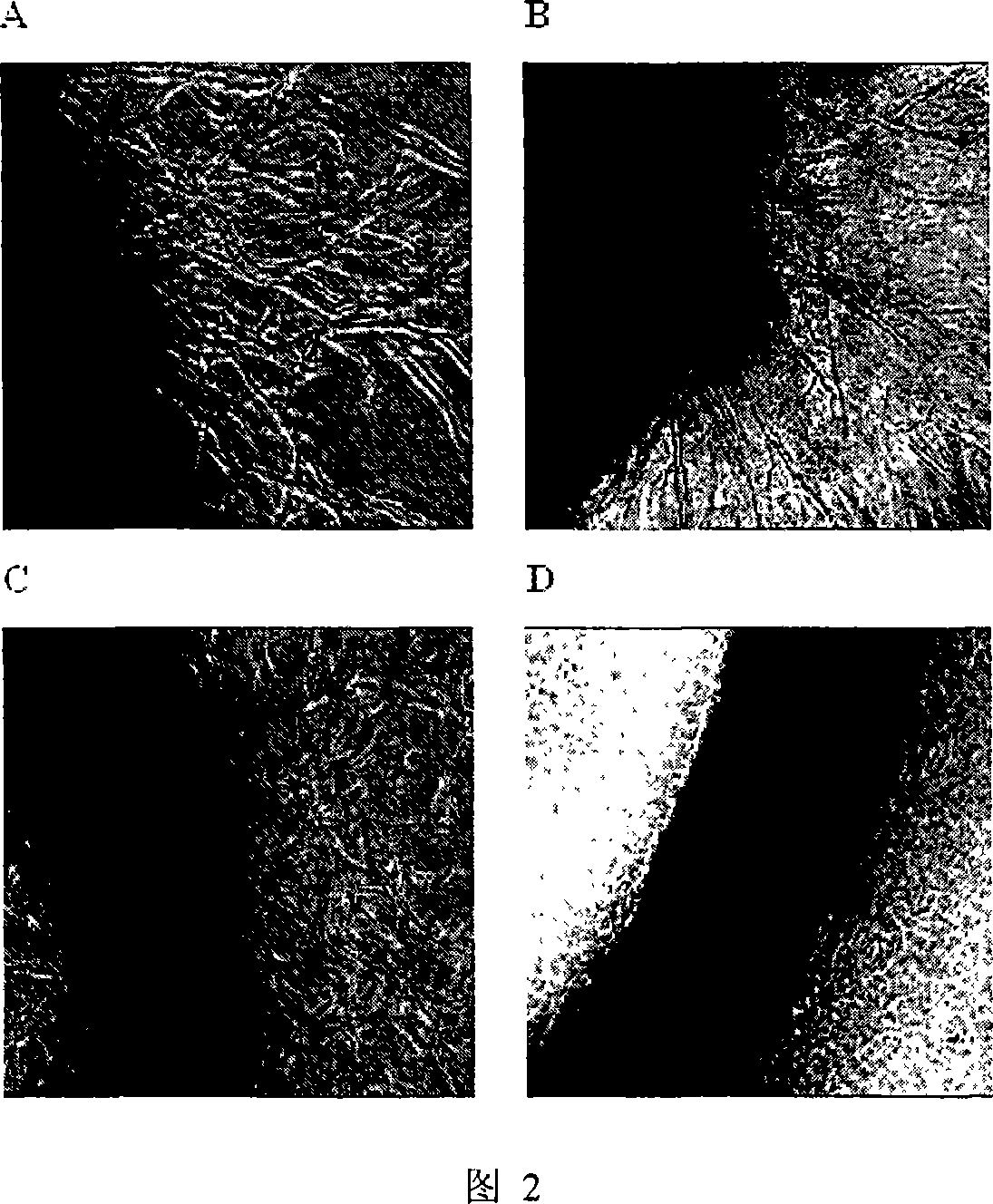
[0024] Example 2: Gambogic acid inhibits angiogenesis of rat arterial rings cultured in vitro.
[0025] Purpose and principle: Rat aortic rings were embedded in a gel matrix to observe endothelial cells budding from the intima and forming capillary-like structures.
[0026] Methods: One male Wistar rat weighing 100-120 g was sacrificed by dislocation, the thoracic aorta was removed, the peripheral connective tissue and adipose tissue were removed, the arterial ring was cut into a 1 mm long ring, and rinsed with M199 serum-free culture medium. The rat aortic segment was embedded in fibrin glue in a 24-well plate, and cultured in vitro with M199 culture solution (control group, without gambogic acid) and culture solution containing different concentrations of gambogic acid. Change the medium once every 3 days.
[0027] RESULTS AND EVALUATION: Endothelial cells grew outward from the arterial incision, forming a branched microvessel-like structure. After 7 days, the formation of...
Embodiment 3
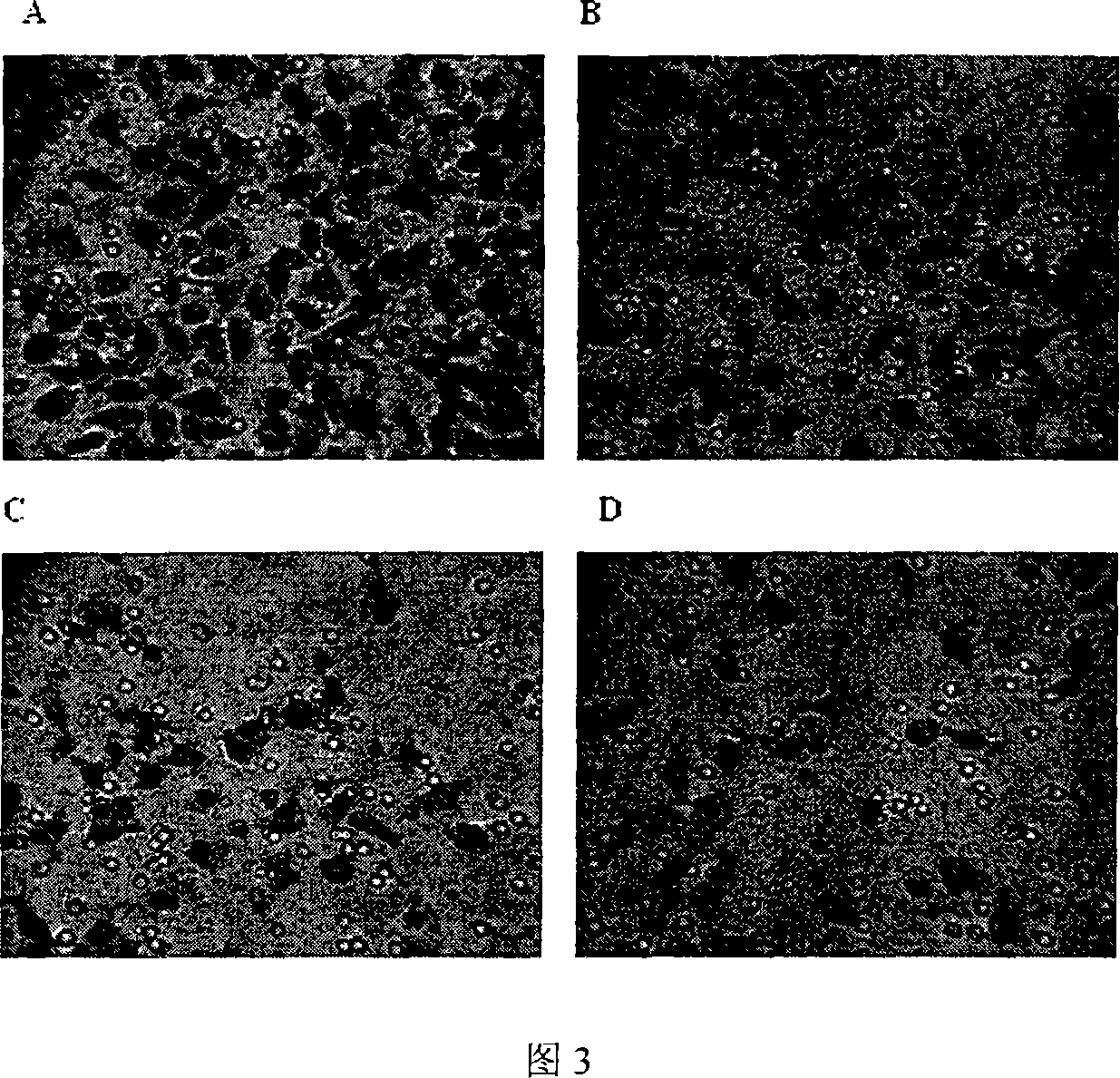
[0028] Example 3: Gambogic acid inhibits migration of endothelial cells.
[0029] Purpose and principle: The endothelial cell migration experiment was carried out in a transwell cell culture chamber. The polycarbonate filter membrane with a pore size of 8 μm separates the chamber into upper and lower layers. Under the stimulation of growth factors, endothelial cells pass through the 8 μm filter pores through amoeba movement and attach to the back of the filter membrane. This model can be used to evaluate the effect of drugs on the migration ability of endothelial cells.
[0030] Method: Endothelial cells were treated with 1640 medium containing different concentrations of gambogic acid for 30 minutes, and then 2.5×10 5 400 μl of cell suspension of each / ml HUVEC was added to the upper chamber, and 600 μl of 1640 culture medium containing stimulatory factors and different concentrations were added to the lower chamber, and placed in 5% CO 2 Incubate in an incubator at 37°C fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com