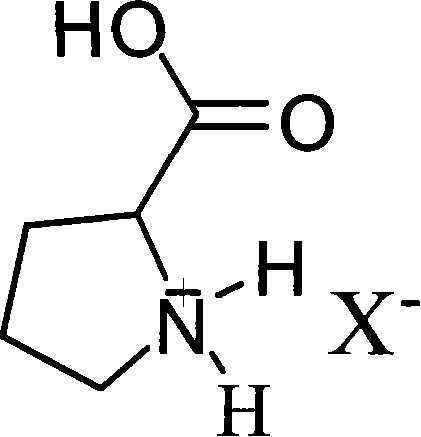
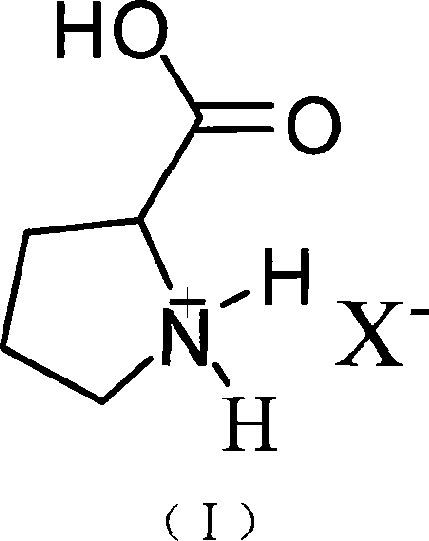
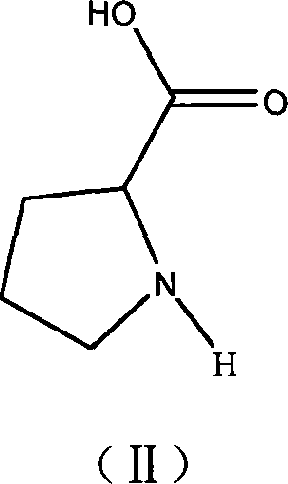
Bronsted acidic compound of containing L- proline radical, preparation method, and application
A Bronsted acid and acidic compound technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., to achieve reduced synthesis costs, high yield, and complete conversion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 11.5 grams (0.1 mol) of L-proline, slowly add 21.95 grams of 40wt% tetrafluoroboric acid aqueous solution (containing 0.1 mol tetrafluoroboric acid) dropwise under stirring at 0°C, drop it in 30 minutes, and continue to stir the reaction at room temperature After 2 hours, heat and remove water under vacuum to obtain the product L-proline fluoroboric acid ionic liquid. Stable to water and air, the yield is 98%. Density 1.156 g / ml, conductivity 1.43×10 -3 S / cm, the melting point is -68°C.
[0024] High resolution mass spectrometry: [M-BF 4 ] + =116
[0025] 1 HNMR (CDCl 3 , δ / ppm relative to TMS): 15.60 (t, 2H), 12.37 (m, 1H), 4.415-4.418 (m, 1H), 3.332 (t, 2H), 1.974 (t, 4H).
Embodiment 2
[0027]Weigh 11.5 grams (0.1mol) of L-proline and dissolve it in water, slowly add 6.3 grams (0.1mol) of 65wt% concentrated nitric acid dropwise under stirring at room temperature, drop it in 30 minutes, continue stirring and reacting for 6 hours at room temperature, heat Water was removed in a vacuum to obtain the product L-proline nitrate ionic liquid. Stable to water and air, the yield is 97.7%. Density 1.323 g / ml, conductivity 2.5×10 -3 S / cm, the melting point is -57°C.
[0028] High resolution mass spectrometry: [M-NO 3 ] + =116
[0029] 1 HNMR (D 2 O, δ / ppm relative to TMS): 4.415-4.418 (m, 1H), 3.332 (t, 2H), 1.974 (t, 4H).
Embodiment 3
[0031] Weigh 11.5 g (0.1 mol) of L-proline and dissolve it in water, slowly add 11.40 g (0.1 mol) of chloroacetic acid dropwise under stirring at room temperature, and finish dropping in 50 minutes, continue to stir and react at room temperature for 6 hours, heat and vacuum to remove water , that is, a brown viscous liquid is obtained, which is the product L-proline chloroacetic acid ionic liquid. Stable to water and air, the yield is 96%. Density 1.134 g / ml, conductivity 2.3×10 -3 S / cm, the melting point is -68°C.
[0032] High resolution mass spectrometry: [M-ClCH 2 COO] + =116
[0033] 1 HNMR (D 2 O, δ / ppm relative to TMS): 4.415-4.418 (t, 3H), 3.332 (t, 2H), 1.974 (t, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com