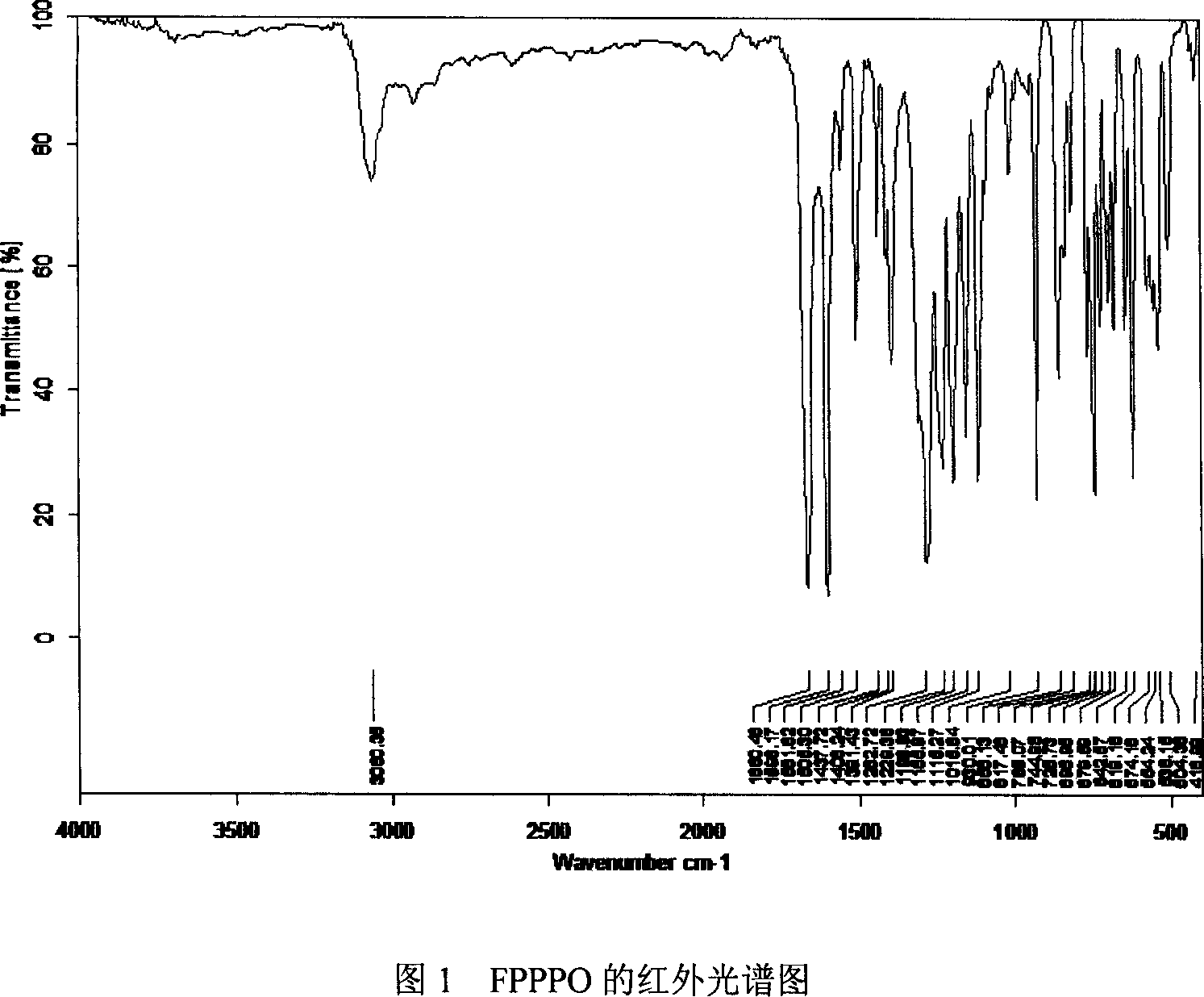
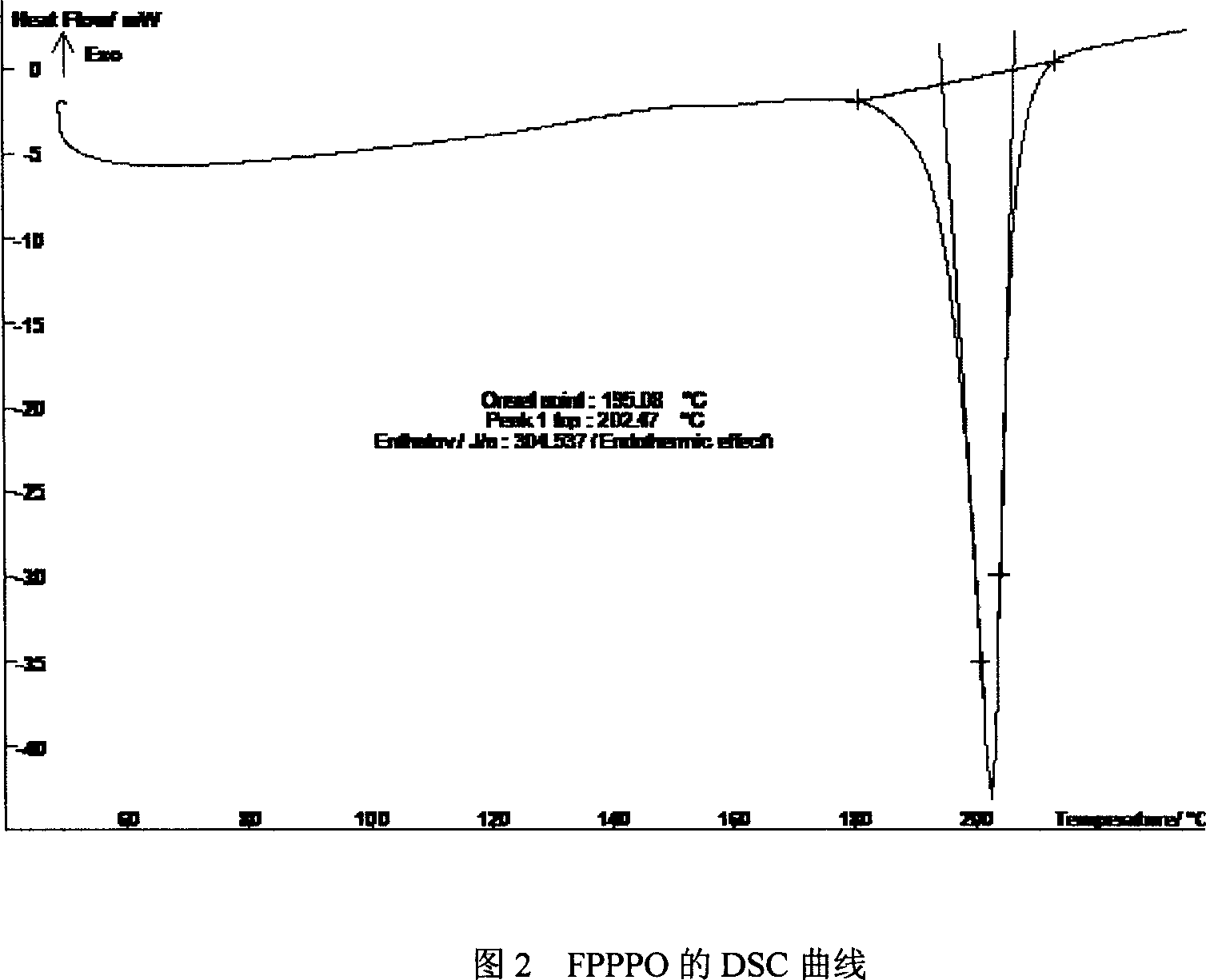
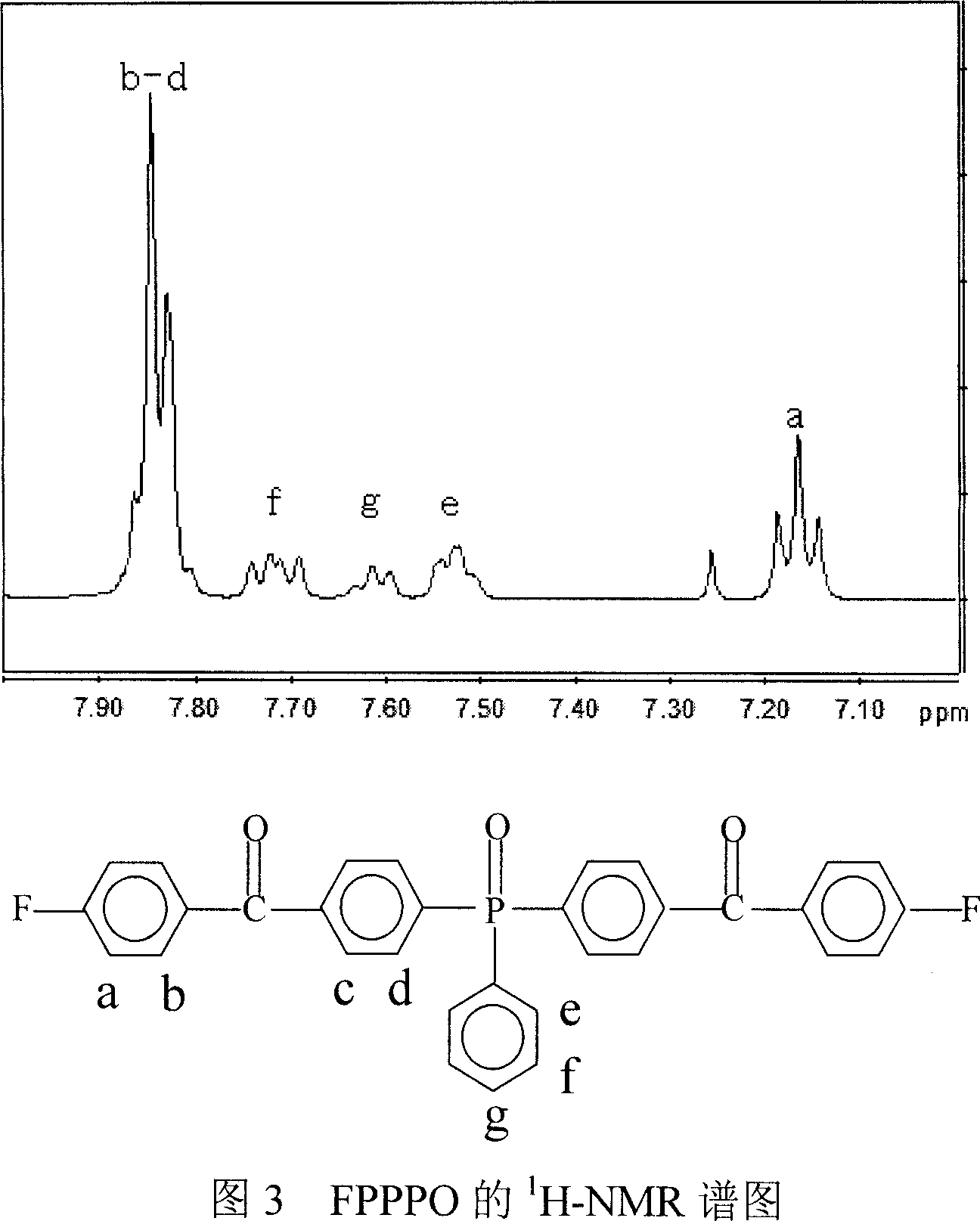
Bi(4-(4-fluorobenzoyl)phenyl)phenyl phosphine oxide monomer and synthetic method thereof
A technology of fluorobenzoyl and phenylphosphine oxide, applied in the field of novel structure phosphorus-containing aromatic difluoride monomer and its synthesis, can solve the problems of high melting temperature of polyaryl ether ketone, difficult processing, insoluble and the like , to achieve the effect of strong repeatability, high monomer yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of CPPPO involved in the present invention
[0023] Add 22.0g (0.06mol) BCPPO, 100ml thionyl chloride and 4-5 drops of catalyst DMF in a 250ml four-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, react at reflux temperature for 6h, and distill under reduced pressure Excessive thionyl chloride was removed, the residual thionyl chloride was washed with n-hexane, and vacuum-dried to obtain 22.8 g of light yellow solid CPPPO, with a yield of 94%.
Embodiment 2
[0024] Embodiment 2: the synthesis of FPPPO involved in the present invention
[0025] Add 24.5g (0.06mol) CPPPO, 24.0g (0.18mol) AlCl 3 and 60ml of dichloromethane, add 15ml (0.16mol) fluorobenzene dropwise with a constant pressure funnel at 40°C, react at this temperature for 5 hours, pour the reactant into 200ml of water to remove AlCl 3 , suction filtration, then the solid product is poured into 200ml 10% sodium hydroxide solution to remove the acidic substance remaining in the product, and the product is washed to neutrality with distilled water, and suction filtration obtains 27.2g light yellow powdery crystals, producing The rate is 86%.
Embodiment 3
[0026] Embodiment 3: the purification of FPPPO involved in the present invention
[0027] Add 25.0g of FPPPO, 75ml of DMF, 250ml of absolute ethanol into a 500ml four-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, add 0.75g of activated carbon, reflux for 60min, and suction filter while it is hot to obtain a light yellow solution. After natural cooling and static crystallization, 22.6 g of white powder crystal FPPPO was obtained by filtration.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com