Preparation method of 3,4-ethene dioxythiophene
A technology of ethylenedioxythiophene and dihydroxythiophene, which is applied in the field of synthesis of organic compounds, can solve the problems of low product yield and high cost of raw materials, and achieve the effects of high product yield, beautiful color and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
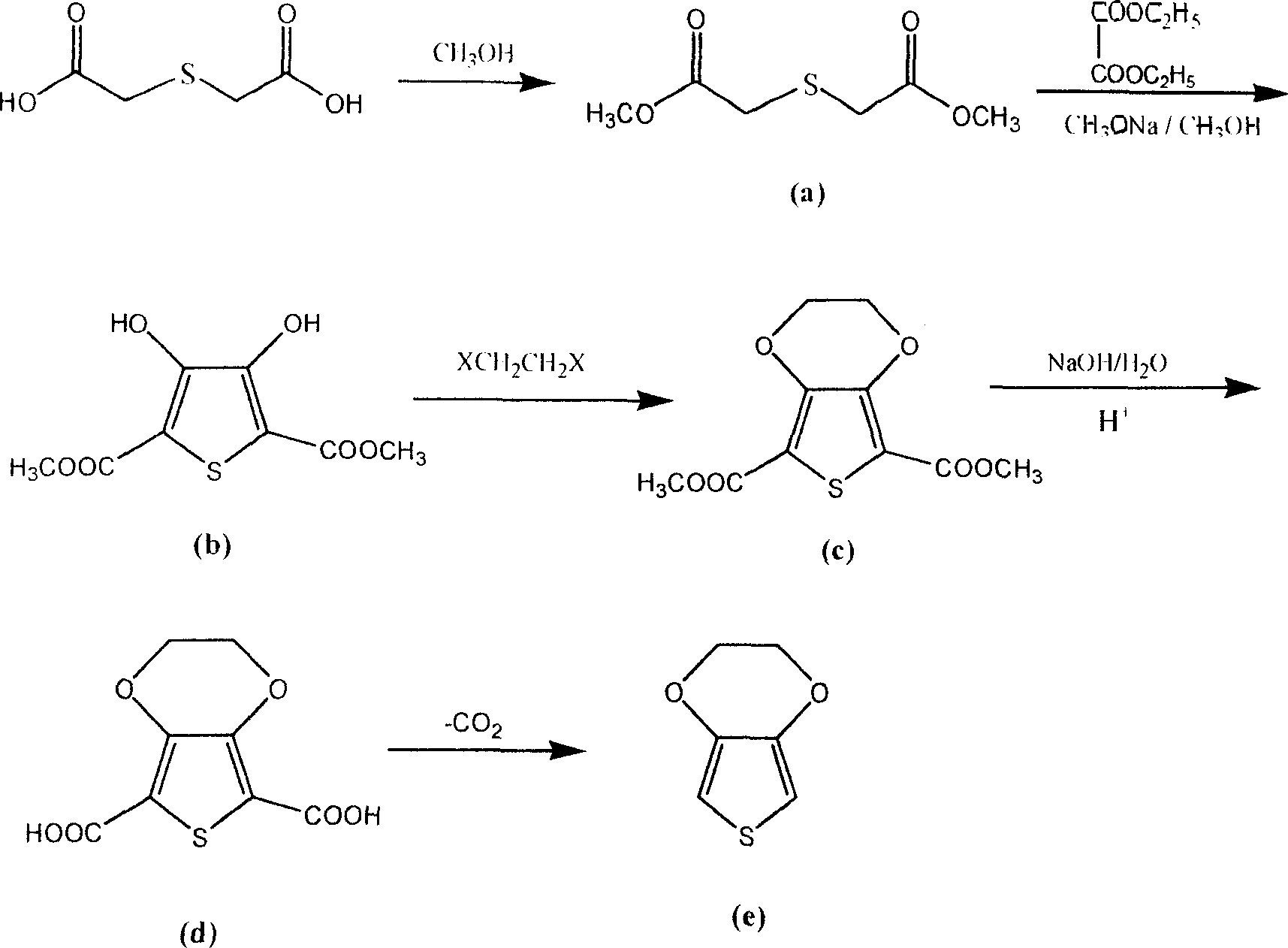
[0032] Mix 338g (2.25mol) of thiodiglycolic acid, 2560ml of methanol, and 135g of sulfuric acid (catalyst), stir, and heat up to reflux. The reaction time is 6-8h. After the reaction is completed, the methanol is distilled out under normal pressure, the temperature is lowered, and 5% lye is added. Adjust the pH to 7-7.5, extract with ether (3*300ml), and dry over anhydrous sodium sulfate. Concentrate diethyl ether under normal pressure, then distill under reduced pressure, collect the distillate with a boiling point of 172-176° C. / 1.33 kPa, and obtain 382.8 g of esterified dimethyl thiodiglycolate, which is a nearly colorless transparent liquid with a content of 99.3%. The rate is 94.8%.
[0033] Catalyst type and consumption are changed, and other is with embodiment 1, and its result is shown in Table 1.
[0034]
[0035] Methanol consumption is changed, and other is with embodiment 1, and its result is shown in Table 2.
[0036] Example
[0037] Reactio...
Embodiment 17
[0041] Add 380g (2.13mol) of the esterified product obtained in Example 1 into 460g (8.52mol) of sodium methoxide in 2475ml of methanol solution, add 772g (5.33mol) of diethyl oxalate dropwise under stirring, and control the temperature at 0-10°C , after the dropwise addition is completed, the temperature is raised to reflux, and the reaction time is 3-5 hours. After the reaction is completed, methanol is recovered, 1L of water is added, acidified with hydrochloric acid, filtered and dried to obtain the condensation product 2,5-dicarboxylic acid of off-white solid powder Dimethyl ester-3,4-dihydroxythiophene 455g, content 99.4%, yield 92%.
[0042] The amount of sodium methylate was changed, and the others were the same as in Example 17. The results are shown in Table 4.
[0043]
[0044] The amount of diethyl oxalate was changed, and others were the same as in Example 17. The results are shown in Table 5.
[0045]
[0046] Preparation of dimethyl 2,5-dica...
Embodiment 26
[0048] With the condensation product 440g (1.9mol) that makes by embodiment 17, 1198g (6.38mol) 1,2-dibromoethane, 503g (3.65mol) K CO , 105g cetyltrimethylammonium bromide (phase transfer catalyst ) into 4500ml of toluene (solvent), stirring and heating up, temperature controlled at 90°C, reaction time 10-15h, after the reaction was completed, the material was concentrated under reduced pressure to recover the solvent and unreacted 1,2-dibromoethane, and added after cooling 5L of water, stirring at room temperature for 30 minutes, followed by suction filtration, washing with water, and drying to obtain the etherification product 2,5-dicarboxylate-3,4-ethylenedioxythiophene 319 as off-white to gray solid powder, with a content of 98.5%. Yield 64%.
[0049] The amount of 1,2-dibromoethane was changed, and the others were the same as in Example 26. The results are shown in Table 6.
[0050]
[0051] Will K 2 CO 3 Consumption changes, and other is with embodiment 26, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com