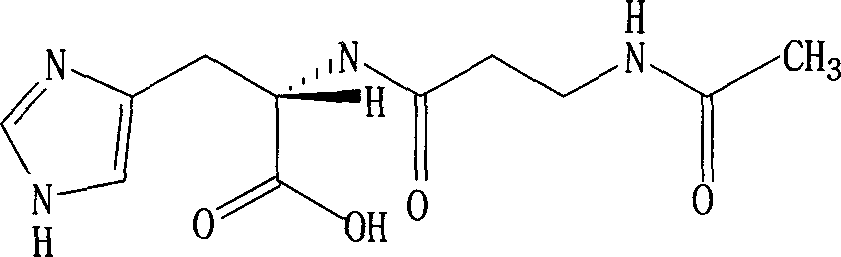
Modified method for chemically synthesizing N-acetyl-L-carnosine
A chemical synthesis and carnosine technology, applied in organic chemistry and other fields, can solve the problems of harmful eyes, racemization, and many steps of histidine methyl ester, and achieve the effect of simple process and guaranteed quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] L-carnosine 90.4g (0.40mol) is dissolved in 200ml 8% sodium hydroxide aqueous solution, under stirring, temperature of reaction must keep on 5-10 ℃, pH is in the scope of 13.0-13.5, drops 95% acetyl chloride 34.5g simultaneously ( 0.44mol) and 260ml of 50% sodium acetate aqueous solution. After completion of the dropwise addition, stir again at the same temperature for 1 hour, and check by HPLC to confirm that the product N-acetyl-L-carnosine is generated, and the disappearance of the raw material and the absence of the by-product N-acetyl-N-(imidazole) acetyl-carnosine . The reaction liquid was treated with strong acidic ion exchange resin SKIB to absorb N-acetyl carnosine, then washed with neutral water, concentrated the original eluate to 200ml with 1mol / L ammonia water, added acetic acid to adjust the pH to 4.9, and kept the temperature at 50 ℃, add 780ml of isopropanol, keep stirring at 50 ℃ for 3 hours, lower to 20 ℃ and stir for another 1 hour, the crude product...
Embodiment 2
[0019] 45.2 g (0.20 mol) of L-carnosine was dissolved in 55 ml of 8% aqueous sodium hydroxide solution, and the pH was adjusted to 13.3, while 20.4 g (0.26 mol) of 95% acetyl chloride and 180 ml of 50% sodium acetate solution were added dropwise. The drop was completed in 1.5 hours, during which the pH of the solution was 12.5-13.5, and the reaction temperature was maintained at 18-20°C. After the dropwise addition, stir at the same temperature for 1 hour, and simultaneously follow up with HPLC to confirm the generation of the reaction object, the raw material and the by-reaction product N-acetyl-N 2 -Acetyl-L was not detected.
[0020] Treat the reaction liquid with strong acid resin according to Example 1, adjust the pH and crystallize with isopropanol to obtain 42.4 g of N-acetyl-L-carnosine, the yield is 80.0%, and the specific rotation [α] 20 D =+26.10° (C=3, water), no racemization confirmed.
Embodiment 3
[0022] 67.8g (0.30mol) of L-carnosine was dissolved in 200ml of water, 200ml of acetone and 166ml of 8% sodium hydroxide solution to adjust the pH to 12.5. The solution was stirred, and the reaction temperature was kept at 10°C. At the same time, 25.9 g (0.33 mol) of 95% acetyl chloride, acetone solution and 50% sodium acetate aqueous solution were added dropwise. The pH of the reaction was maintained at 11.5-12.5 during the dropwise addition. After the dropwise addition, stir at room temperature for 30 minutes, evaporate acetone under reduced pressure, add 200 ml of water, and treat N-acetyl-L-carnosine as in Example 1 to obtain 76.5 g of crystals with a yield of 77.5% and a melting point of 216-218°C. Specific rotation [α] 20 D =+26.0° (C=3, water), no racemization confirmed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com