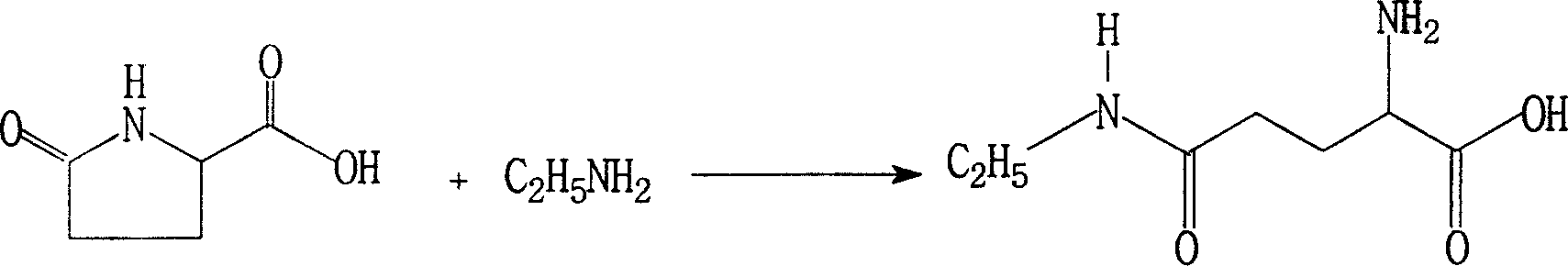
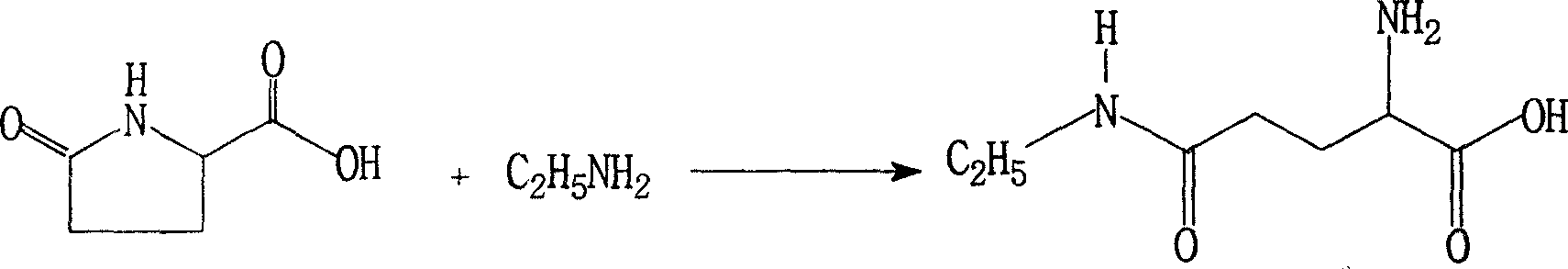
Modified synthesis method for L-theanine
A synthesis method and technology of theanine, applied in chemical instruments and methods, preparation of aminohydroxy compounds, preparation of carboxylic acid amides, etc., can solve the problems of side reaction products, deterioration of color, and reduction of yield, etc., to avoid oxidation The effect of reaction, convenient operation and short process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
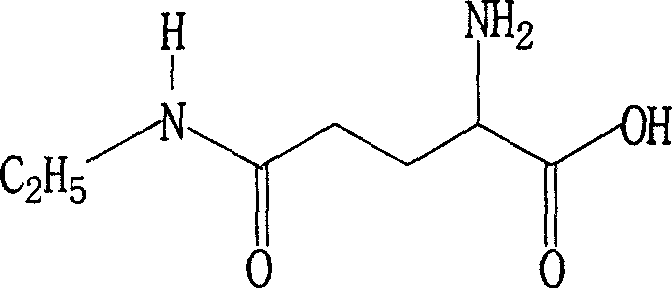
[0030] In a 500L stainless steel autoclave, add 60Kg of L-pyrrolidone acid and 0.6Kg of ethoxyquinoline, seal the autoclave, add 200Kg of liquid anhydrous ethylamine, heat slowly with a hot water bath, and keep the internal temperature at 30-59°C. Stir at a medium speed, keep the pressure at 0.4-5.9MPa, react for 48 hours, and when the reaction is over, recover excess ethylamine, which can be reused for feeding. The reaction product was decolorized with activated carbon and recrystallized from 95% ethanol to obtain 47.3Kg of refined product, yield: 58.5%, [α] 20 D +7.7~+8.5°(5%, H 2 O), purity: (HPLC) ≥ 98.5%.
Embodiment 2
[0032] In a 500L stainless steel autoclave, add 60Kg of L-pyrrolidone acid to a closed reaction autoclave, exhaust the air in the autoclave with dry ethylamine gas in the steel cylinder, then add 190Kg of liquid anhydrous ethylamine, slowly heat with a hot water bath, Keep the temperature at 30-59°C, stir at a medium speed, and keep the pressure at 0.4-5.9MPa. React for 60 hours. After the reaction is over, recover excess ethylamine, which can be reused for feeding. The reaction product was decolorized with activated carbon, recrystallized with 95% ethanol, and soaked in absolute ethanol to obtain a fine product of 38.9Kg, yield: 48.2%, [α] 20 D +7.7~+8.5°(5%, H 2 O), purity: (HPLC) ≥ 98.5%.
Embodiment 3
[0034] In a 1000L stainless steel autoclave, add 60Kg L-pyrrolidone acid and 0.6g quinoline as an antioxidant, seal the autoclave, exhaust the air in the autoclave with the dry gas in the steel cylinder, then add 380Kg of liquid anhydrous ethylamine, heat Slowly heat the water bath, keep the internal temperature at 30-59°C, stir at a medium speed, keep the pressure at 0.4-5.9MPa, react for 48 hours, the reaction crystallizes, recover excess ethylamine, and the recovered ethylamine can be fed repeatedly and used mechanically. The reaction product was decolorized with activated carbon, recrystallized from 95% ethanol, and 97.2Kg of fine product was obtained, yield: 60.1%, [α] 20 D +7.8~+8.5°(5%, H 2 O), purity: (HPLC) ≥ 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com