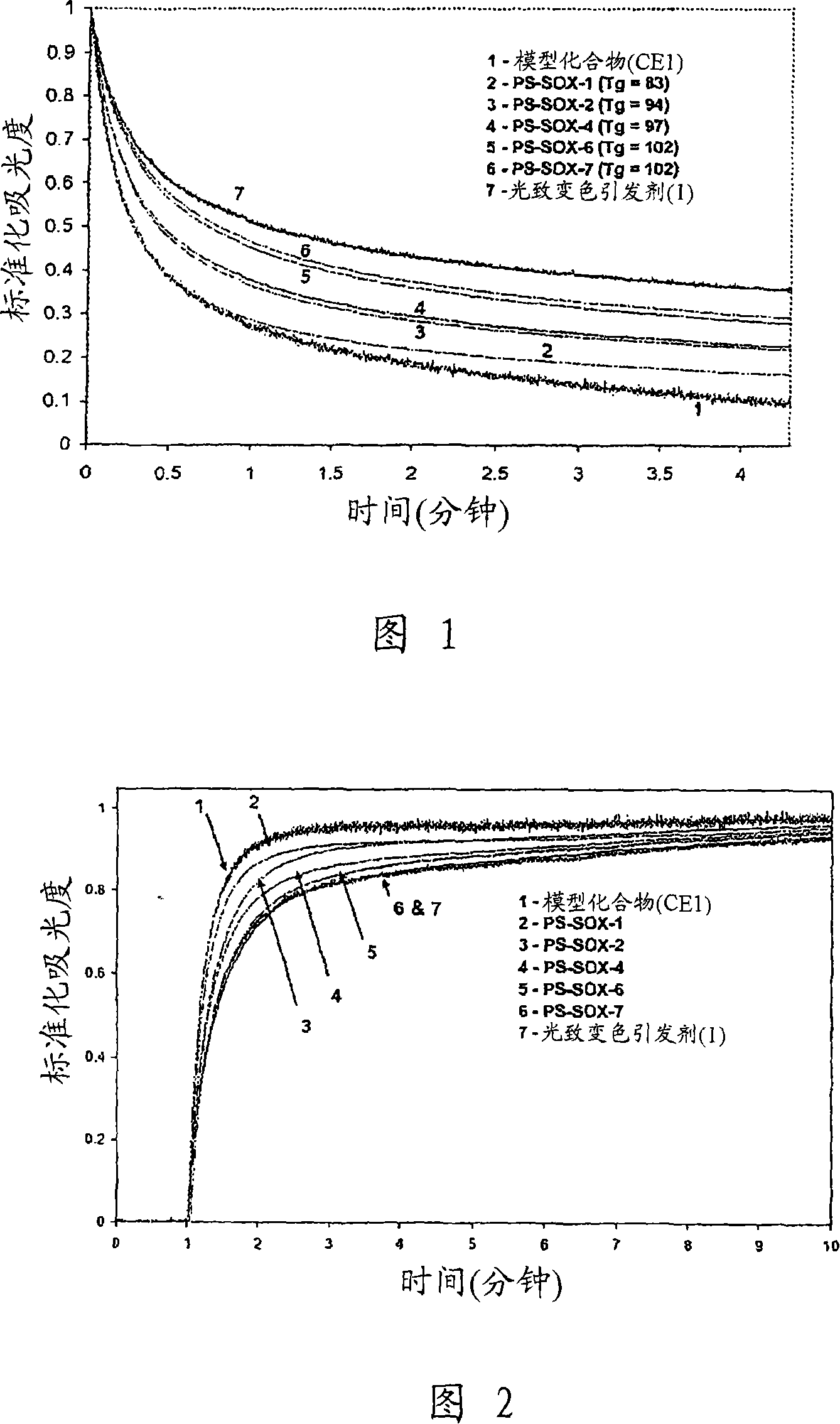
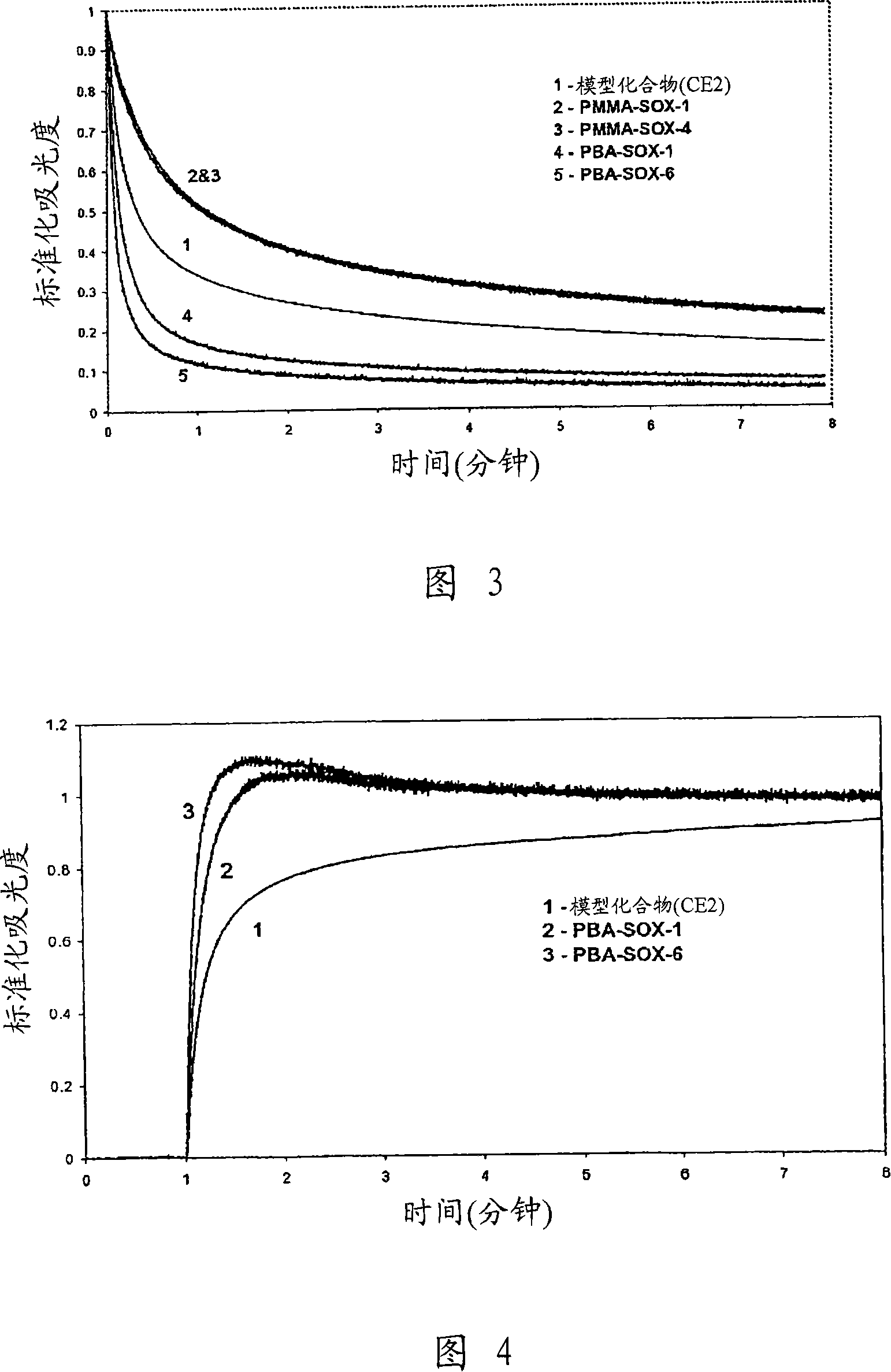
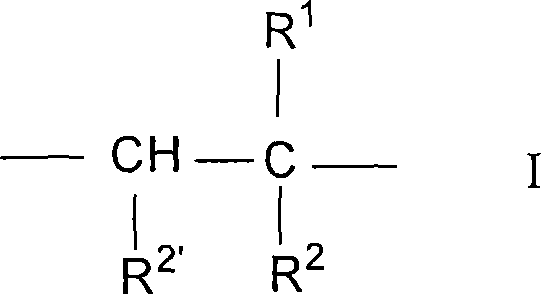
Photochromic compounds comprising polymeric substituents and methods for preparation and use thereof
A photochromic and compound technology, applied in the field of polymer products that can transmit light, can solve problems such as increasing the fading speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0472] Synthesis of Photochromic Initiator 1 and Spirooxazine-Polystyrene Polymer Conjugates
[0473] Synthesis of the first step photochromic initiator 1
[0474]
[0475] 9'-(4-Chloromethylbenzoyloxy)-1,3,3-trimethylspiro[indoline 2,3'-[3H]naphtho[2,1-b][1 , 4] oxazine (1)
[0476] 9'-Hydroxy-1,3,3-trimethylspiro[indoline 2,3'-[3H]naphtho[2,1-b][1,4]oxazine (2.5g, 72.7 ×10 -4 mol) into 30 mL of dichloromethane in a 100 mL three-neck round bottom flask. Add triethylamine (1.10g, 10.9×10 -3 mol), and the reaction mixture was stirred for half an hour. Then 4-(chloromethyl)benzoyl chloride (1.65g, 87.2×10 -4mol) was dissolved in 10 mL of dichloromethane and added dropwise to the reaction mixture under argon, cooled to 0 °C. The reaction mixture was stirred for a further 1 hour at freezing temperature and then for a final hour at room temperature. The obtained product was confirmed by TLC with 1:1 diethyl ether and hexane. Wash the product with 100 mL 0.5M NaOH, 100 m...
Embodiment 2
[0487] Example 2 Synthesis of Photochromic Initiator 2
[0488]
[0489] 9'-(4-methylbenzoyloxy)-1,3,3-trimethylspiro[indoline 2,3'-[3H]naphtho[2,1-b][1, 4] Oxazine (2)
[0490] This compound was synthesized as in Example 1, but the acid chloride used was 2-bromo-isobutyl bromide. The product is purified by column chromatography. 1 H NMR ((CD 3 ) 2 CO) δ: 1.34, 1.36, 2.16, 2.78, 6.67, 6.87, 7.08, 7.16, 7.20, 7.36, 7.84, 7.86, 7.96, 8.38 ppm.
Embodiment 3
[0492] Synthesis of Photochromic Initiator 3
[0493]
[0494] Butane-1-thiol (1.06g, 1.17×10 -2 mol), carbon disulfide (2.14g, 2.81×10 -2 mol) and triethylamine (2.85g, 2.81×10 -2 nol) was added to 30 mL of chloroform and stirred overnight. Subsequently, 9'-(4-chloromethylbenzoyloxy)-1,3,3-trimethylspiro[indoline 2,3'-[3H]naphtho[2,1-b ][1,4]oxazine](5.82g, 1.17×10 -2 mol), and stirred at 60° C. until TLC (3:2 diethyl ether and hexane) confirmed the formation of the product. The product was purified by washing with water, brine, and drying over magnesium sulfate, followed by recrystallization from methanol / chloroform. 1 H NMR ((CD 3 ) 2 CO) δ: .94, 1.35, 1.36, 1.47, 1.71, 2.78, 3.46, 4.85, 6.67, 6.87, 7.07, 7.16, 7.20, 7.37, 7.68, 7.84, 7.86, 7.95, 8.23, 8.40ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com