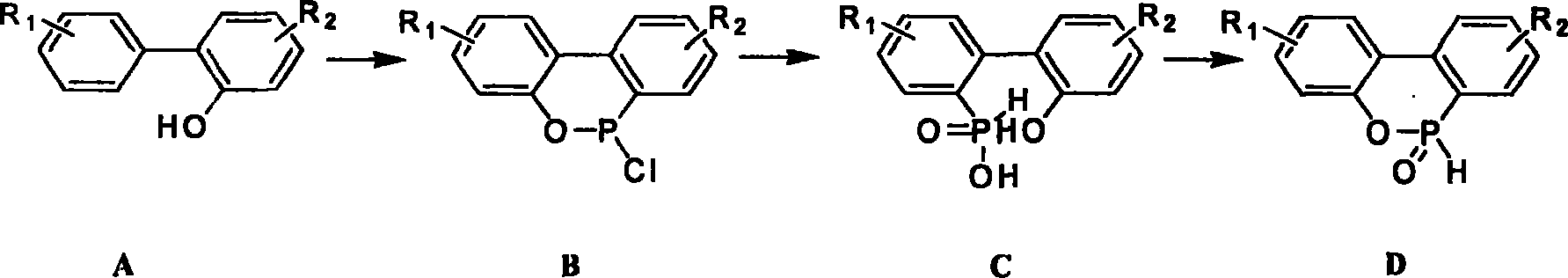
Prepn process of cyclic phosphonate compound
A technology for cyclic phosphonates and compounds, which is applied in the field of preparation of cyclic phosphonate compounds, can solve the problems of oxide organic phosphoric acid impurities, increase production costs, uneven heating of materials, etc., achieves fast reaction speed and simplifies processes Difficulty, good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
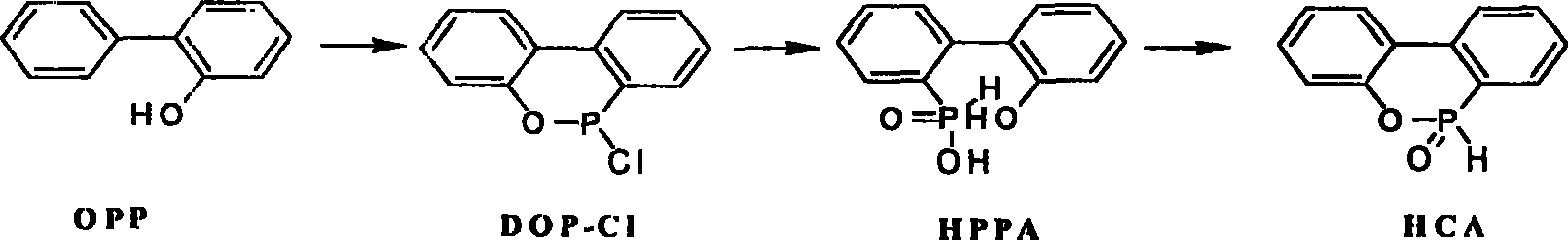
[0015] Example 1 Methanol is used as solvent intramolecular dehydration ring-closing to synthesize HCA
[0016]
[0017] (1). Preparation of HPPA
[0018] Add 1400 kg of OPP, 1200 kg of phosphorus trichloride, and 25 kg of zinc chloride into a 5000-liter reactor, slowly raise the temperature to 70°C for reflux, then gradually raise the temperature to 120-165°C, and maintain the reaction for 10-30 hours. Colorless to orange is the end of the reaction. After the reaction, the temperature was lowered to 60-100° C., and 1600 kg of toluene was added to dissolve the generated DOP-Cl. The insoluble matter was removed by filtration. Add 400 kg of water dropwise to the filtrate for hydrolysis, the hydrolysis reaction is carried out between 30-80°C, and it takes about 2 hours to complete. After the reaction, the material was down to 25° C., crystallized, and centrifuged to obtain 1967 kg of HPPA wet product with a solid content of 85%. The HPLC (normalization method) analysis prod...
example 2
[0024] Example 2 ethanol as solvent intramolecular dehydration ring-closure synthesis of HCA
[0025] Feeding and operating steps are the same as example 1, and the solvent is replaced with 150 kilograms of ethanol. Obtain 254 kg of white flake product, purity: 99.1%, yield 93.6%. Melting point: 118-119°C, OPP content 0.03%, chloride ion content 30ppm.
example 3
[0026] Example 3 water as solvent intramolecular dehydration ring-closing synthesis of HCA
[0027] Feeding and operation steps are the same as example 1, and solvent is replaced with 150 kilograms of water. 255 kg of white flakes were obtained with a purity of 99.2% and a yield of 94.0%. Melting point: 118-119°C, OPP content 0.01%, chloride ion content 30ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com