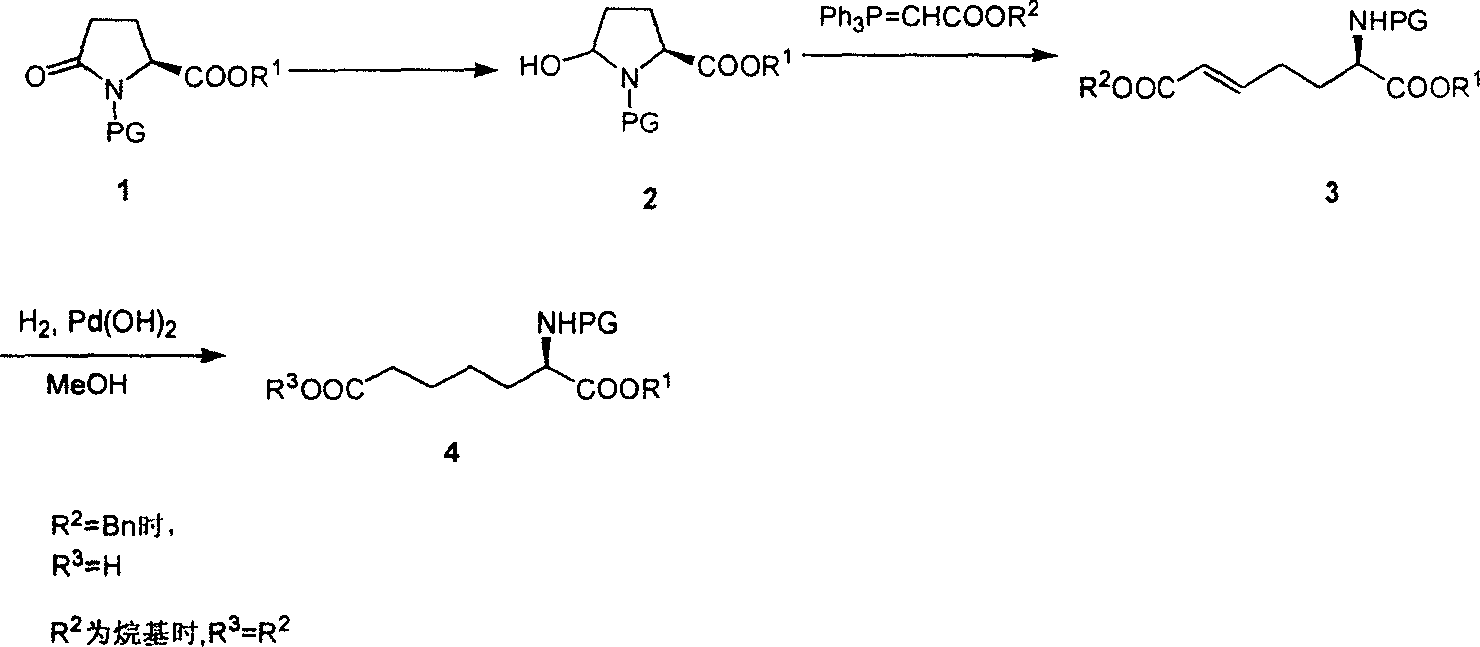
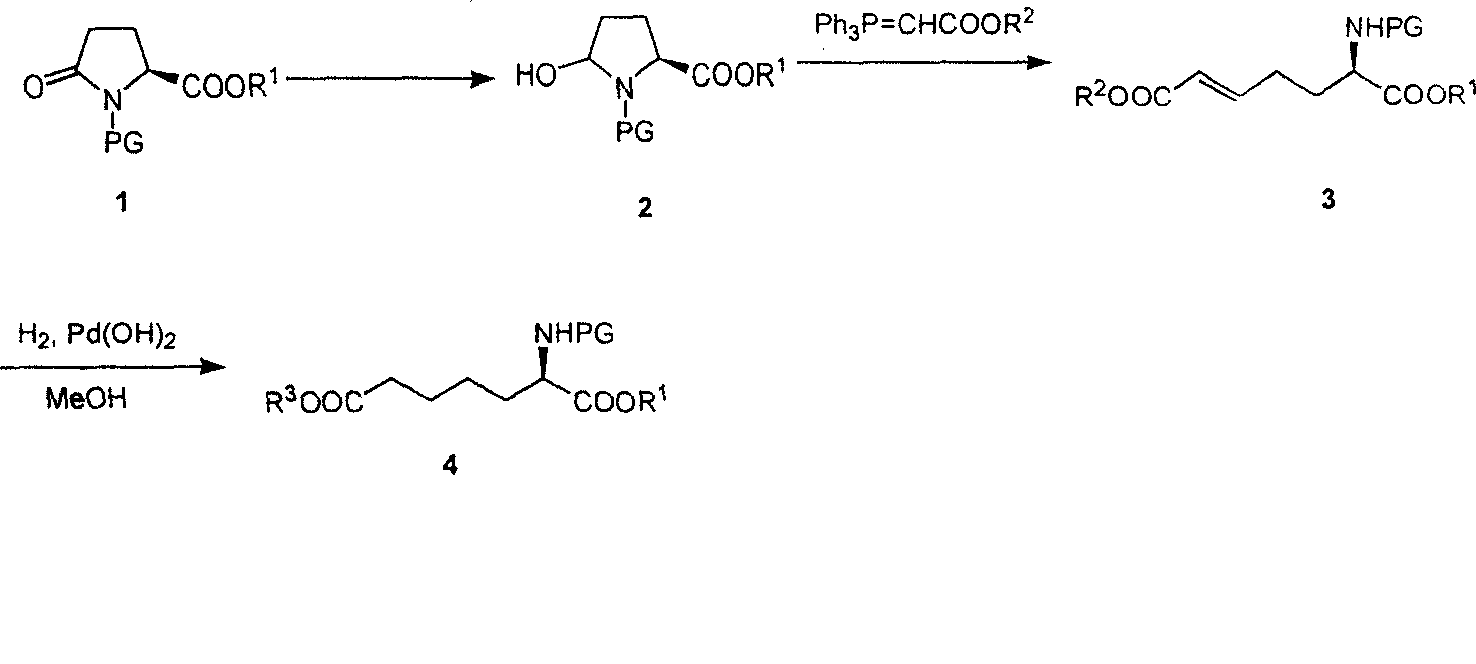
Method for practical synthesizing optically active alpha amino pimelic acid ester or monoester
An aminopimelic acid ester, optically active technology, applied in chemical instruments and methods, organic chemistry, bulk chemical production, etc., can solve the problems of unsuitability for industrial production, harsh reaction conditions, and low utilization of raw materials, and achieve easy Effects of magnification, easy access to raw materials, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1. Synthesis of 5-Hydroxy-N-Boc-prosthenic acid ethyl ester
[0017]
[0018] Dissolve L-N-Boc-ethyl pyroglutamate 1 (30 g, 117 mmol) in anhydrous THF, cool to -78°C in a dry ice-acetone bath, and slowly add DIBAL-H toluene solution (1 M, 233mL, 233mmol), the rate of addition was controlled so that the addition was completed within 1 hour. After the dropwise addition, the reaction solution was continuously stirred for 45 minutes and monitored by TLC. After the reaction was complete, isopropanol was slowly added to quench the reaction, then an aqueous solution of potassium sodium tartrate was added, and the reaction solution was continuously stirred for 20 minutes. The layers were left to stand, the aqueous phase was extracted with ether, the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 After drying and concentration, 5-hydroxy-N-Boc-proline ethyl ester 2 (29.5 g, 97.6%) was obtained, which was directly put into the next reaction withou...
Embodiment 2
[0029] 1. Synthesis of 5-Hydroxy-N-Boc-prosthenic acid ethyl ester
[0030]
[0031] Dissolve L-N-Boc-ethyl pyroglutamate 1 (30g, 117mmol) in anhydrous THF, cool to -78°C in a dry ice-acetone bath, slowly add DIBAL-H toluene solution (1M, 233mL, 233mmol), control the rate of addition to make it dropwise in 1 hour. After the dropwise addition, the reaction solution was continuously stirred for 45 minutes and monitored by TLC. After the reaction was complete, isopropanol was slowly added to quench the reaction, then an aqueous solution of potassium sodium tartrate was added, and the reaction solution was continuously stirred for 20 minutes. The layers were left to stand, the aqueous phase was extracted with ether, the organic phases were combined, washed with saturated brine, anhydrous MgSO 4 After drying and concentration, 5-hydroxy-N-Boc-proline ethyl ester 2 (29.5 g, 97.6%) was obtained, which was directly put into the next reaction without further purification.
[0032]...
Embodiment 3
[0042] 1. Synthesis of 5-Hydroxy-N-Boc-prosthenic acid ethyl ester
[0043]
[0044] Dissolve L-N-Boc-ethyl pyroglutamate 1 (10g, 39mmol) in anhydrous THF, cool to -78°C in a dry ice-acetone bath, then slowly add LiBEt 3 H solution in THF (1M, 47mL, 47mmol), the rate of addition was controlled so that the addition was completed within 10 minutes. After the dropwise addition, the reaction solution continued to stir for 30 minutes, and was monitored by TLC. After the reaction was complete, slowly added saturated NaHCO 3 Quenches the reaction. After warming up to 0°C, slowly add 30% H 2 o 2 , continued to stir for 30 minutes, concentrated under reduced pressure, the residue was diluted with EtOAc, washed with saturated brine, and the aqueous phase was extracted twice with EtOAc. Mixed organic phase, Na 2 SO 4 Dry, filter, and concentrate the filtrate to obtain 5-hydroxy-N-Boc-proline ethyl ester.
[0045] 2. Synthesis of diethyl L-2-aminohept-5-enedioic acid
[0046] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com