Isosorbide mononitrate orally disintegrating tablets
A technology of isosorbide dinitrate and orally disintegrating tablets, which can be used in pill delivery, cardiovascular system diseases, and devices for making drugs into special physical or ingestion forms, and can solve the problems of poor hardness and integrity of tablets, production conditions, etc. High, need special packaging and other problems, to achieve the effect of overcoming disintegration, high dissolution release, fast disintegration and dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
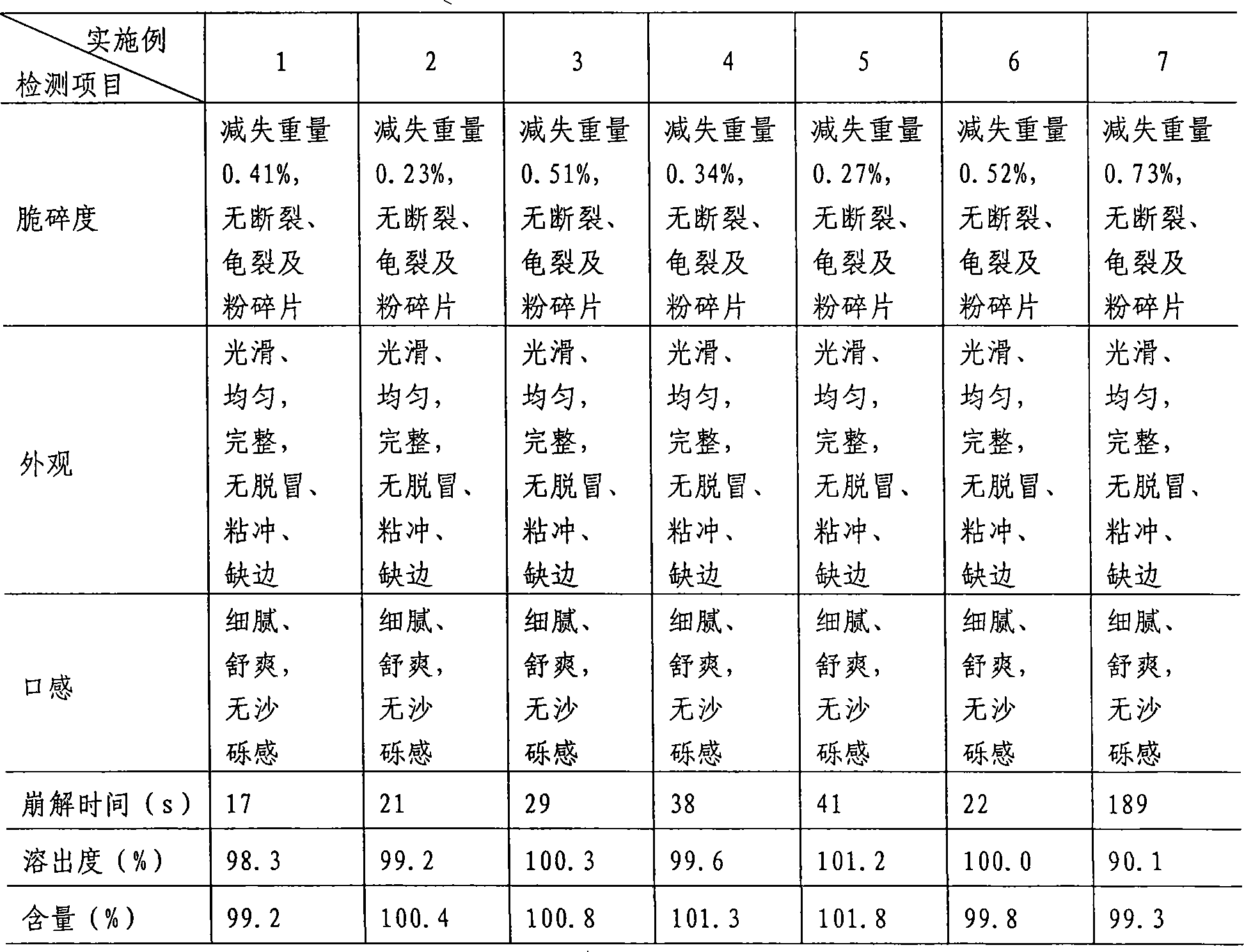
Examples
Embodiment 1
[0029] Embodiment 1 melting hot extrusion method
[0030] Prescription: Isosorbide Mononitrate 10g
[0031] Macrogol 6000 10g
[0032]Mannitol 55g
[0033] Microcrystalline Cellulose 25g
[0034] Low-substituted hydroxypropyl cellulose 5g
[0035] Croscarmellose Sodium 5g
[0036] Cross-linked polyvinylpyrrolidone 5g
[0037] Stevia 3g
[0038] Micronized silica gel 1g
[0040] Makes 1000 pieces
[0041] Preparation method: Take the prescription amount of isosorbide mononitrate and add it to the prescription amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and use a melting heat extruder under negative pressure Take the molten mixture material, cool it down to 40°C under gradient cooling, extrude it with compressed air, granulate it through a sieve plate with a rotary swinging granulator, cool and solidify rapidly at 0°C, and then freeze it at -10°C 2 hours, take it out, dry, ...
Embodiment 2
[0042] Embodiment 2 melt hot extrusion method
[0043] Prescription: Isosorbide Mononitrate 10g
[0044] Macrogol 6000 5g
[0045] Mannitol 50g
[0046] Microcrystalline Cellulose 30g
[0047] Low-substituted hydroxypropyl cellulose 10g
[0048] Croscarmellose Sodium 5g
[0049] Cross-linked polyvinylpyrrolidone 10g
[0050] Stevia 1g
[0051] Micronized silica gel 2g
[0052] Magnesium stearate 0.5g
[0053] Makes 1000 pieces
[0054] Preparation method: Take the prescription amount of isosorbide mononitrate and add it to the prescription amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and use a melting heat extruder under negative pressure Take the molten mixture material, cool it down to 35°C under gradient cooling, extrude it with compressed air, and granulate it through a sieve plate with a rotary swinging granulator. Freeze for 1 hour, take it out, dry, crush, pass through an 80 mesh sieve, and pass ...
Embodiment 3
[0055] Embodiment 3 melting hot extrusion method
[0056] Prescription: Isosorbide Mononitrate 10g
[0057] Macrogol 6000 15g
[0058] Mannitol 40g
[0059] Microcrystalline Cellulose 35g
[0060] Low-substituted hydroxypropyl cellulose 10g
[0061] Croscarmellose Sodium 10g
[0062] Cross-linked polyvinylpyrrolidone 10g
[0063] Stevia 1g
[0064] Micronized silica gel 1.5g
[0066] Makes 1000 pieces
[0067] Preparation method: Take the prescription amount of isosorbide mononitrate and add it to the prescription amount of polyethylene glycol 6000 heated at 90-100°C to melt, stir well and evenly to form a molten mixture, and use a melting heat extruder under negative pressure Take the molten mixture material, cool it down to 30°C under gradient cooling, extrude it with compressed air, granulate it through a sieve plate with a rotary swinging granulator, cool and solidify rapidly at -3°C, and then cool it at -10°C Freeze for 3 hours, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com