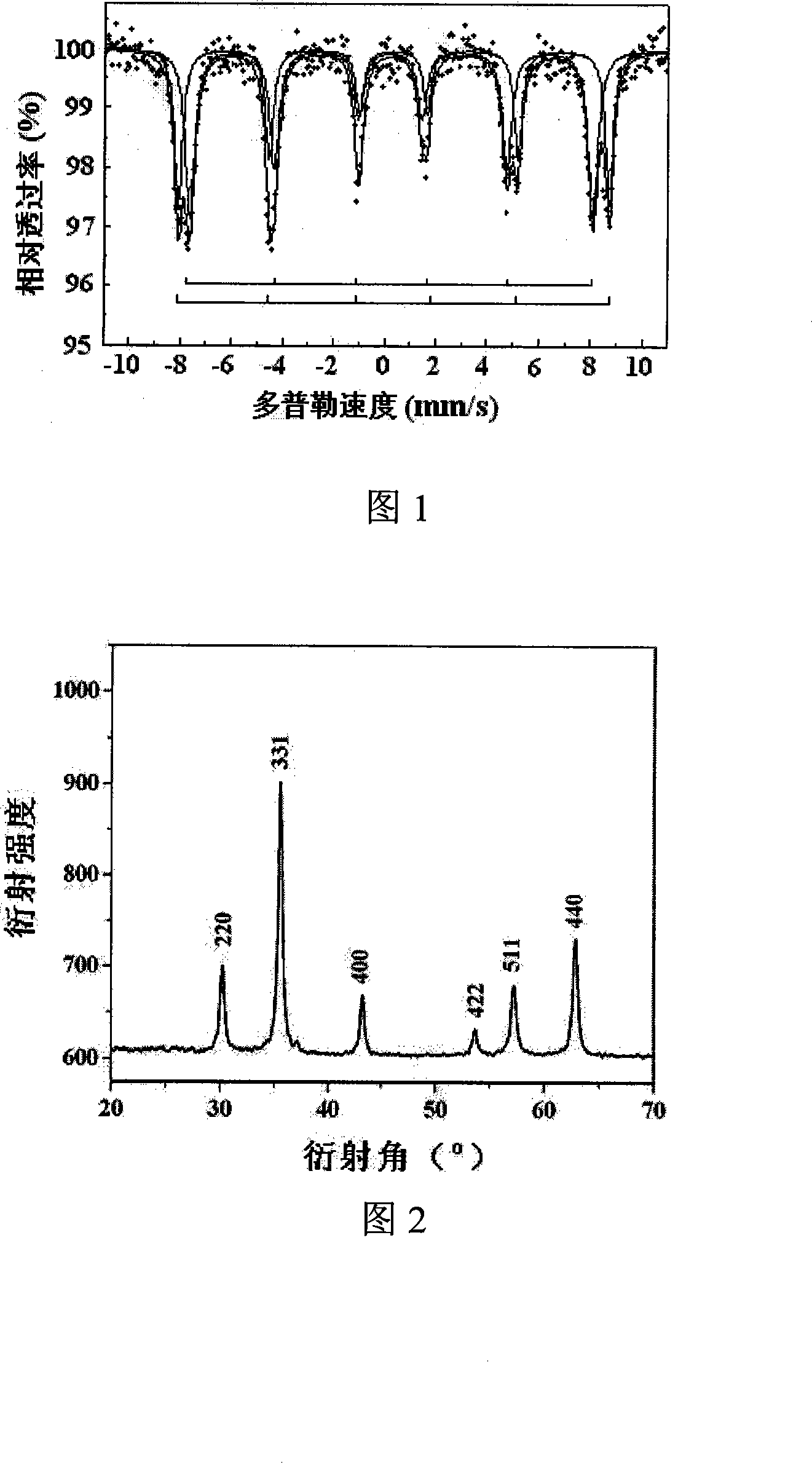
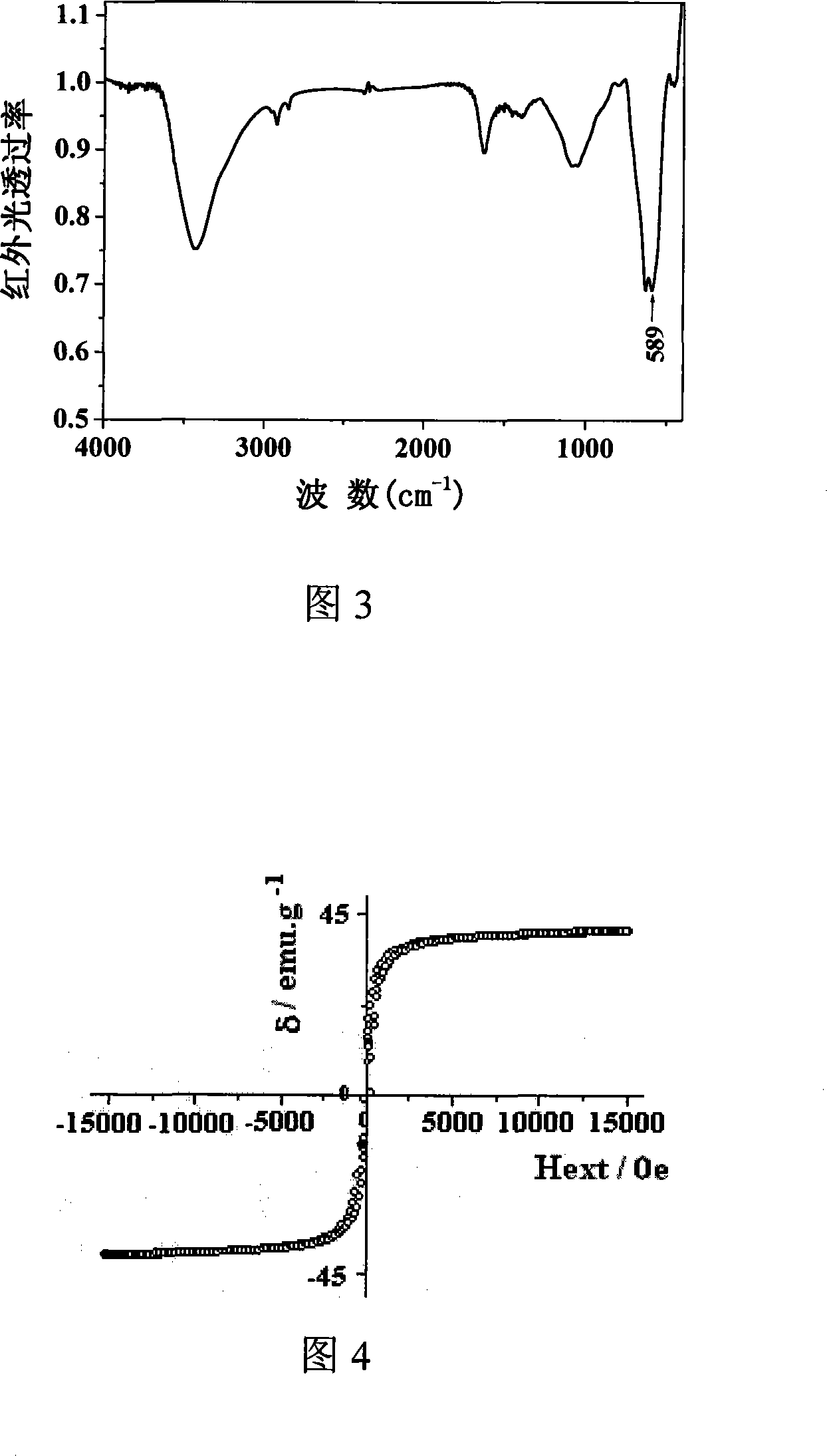
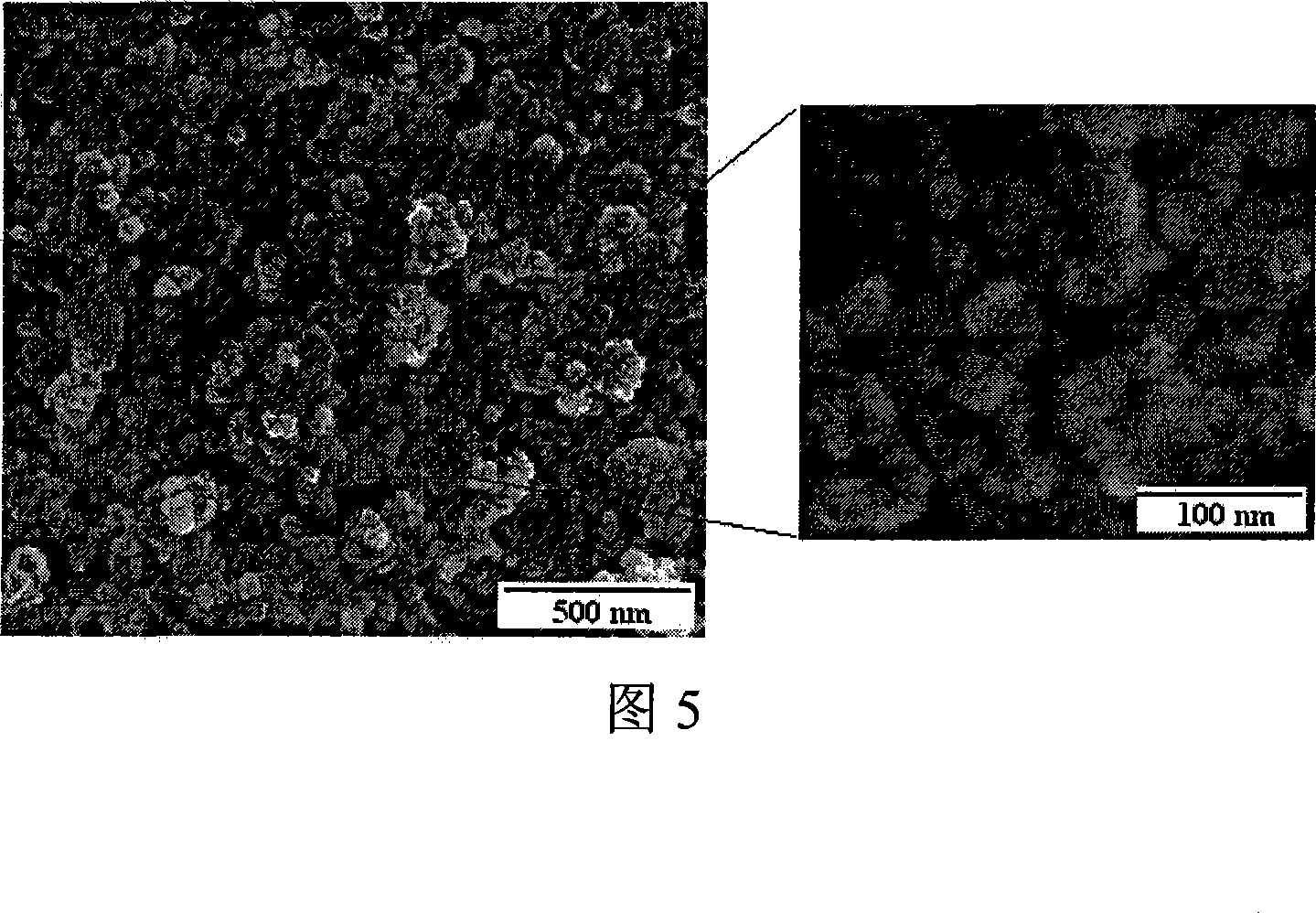
Method for preparing spinel type ferrite under mild condition
A technology of spinel type and ferrite, which is applied in the field of preparation of spinel type ferrite, can solve the problems of difficult control of microemulsion process operation, harsh operating conditions, environmental pollution, etc., and achieve low equipment requirements, Easy to operate and no pollution to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A. Weigh 8.461 grams of Mg(NO 3 ) 2 ·6H 2O, 9.174 g FeSO 4 ·7H 2 O and 6.600 g Fe 2 (SO 4 ) 3 Place in a three-necked flask, add deoxygenated deionized water and stir until the solid mixture is fully dissolved. Preparation of NaOH and NaCO with deoxygenated deionized water 3 A mixed alkaline solution in which the concentration of NaOH is 2.0M, NaCO 3 The concentration is 1.5M. Under nitrogen protection and vigorous stirring, the mixed lye was slowly added dropwise to the salt solution until the pH of the reaction system was ≈11, and then the stirring was continued for 2 hours.
[0032] B. After the stirring is completed, the obtained product is left to stand for crystallization at room temperature for 4 hours, and then placed in a water bath at a constant temperature of 90° C. for crystallization for 18 hours.
[0033] C. Filter the above mixture, and wash with deionized water and absolute ethanol several times successively.
[0034] D. Put the filtered solid...
Embodiment 2
[0038] A, weigh 4.231 grams of Mg (NO 3 ) 2 ·6H 2 O, 4.595 g FeSO 4 ·7H 2 O and 3.300 g Fe 2 (SO 4 ) 3 Place in a three-necked flask, add deoxygenated deionized water and stir until the solid mixture is fully dissolved. Prepare NaOH and NaCO with deoxygenated deionized water 3 A mixed alkaline solution in which the concentration of NaOH is 1.5M, NaCO 3 The concentration is 1.0M. Under nitrogen protection and vigorous stirring, the lye was slowly added dropwise to the above-mentioned salt solution until the pH ≈ 10.5, and then continued to stir for 2 hours.
[0039] B. After the stirring is completed, the product is left to stand for crystallization at room temperature for 5 hours, and then placed in a water bath at a constant temperature of 80° C. for crystallization for 20 hours.
[0040] C. Filter the above mixture, and wash it several times with deionized water and absolute ethanol successively.
[0041] D. Put the filtered solid in a conical flask, add an approp...
Embodiment 3
[0045] A. Weigh 4.075 grams of Co(NO 3 ) 2 ·6H 2 O, 3.892 g FeSO 4 ·7H 2 O and 2.828 g Fe 2 (SO 4 ) 3 Place in a three-necked flask, add deoxygenated deionized water and stir until the solid mixture is fully dissolved. Prepare NaOH and NaCO with deoxygenated deionized water 3 A mixed alkaline solution in which the concentration of NaOH is 2.5M, NaCO 3 The concentration is 1.8M. Under nitrogen protection and vigorous stirring, the mixed lye was slowly added dropwise to the above-mentioned three-neck flask containing the salt solution until pH ≈ 11, and then continued to stir for 2 hours.
[0046] B. After the stirring is completed, the product is left to stand for crystallization at room temperature for 4 hours, and then placed in a water bath at a constant temperature of 95° C. for crystallization for 20 hours.
[0047] C. Filter the above mixture, and wash it several times with deionized water and absolute ethanol successively.
[0048] D. Put the filtered solid in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com