Tamsulosin hydrochloride cotrolled-releasing tablet preparation and preparing method thereof
A technology of controlled-release tablets and tamsulosin, which is applied in the field of pharmaceutical preparations, can solve the problems of PEO not having ideal thermal stability, reducing the tableting speed, and difficulty in solvent drying.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
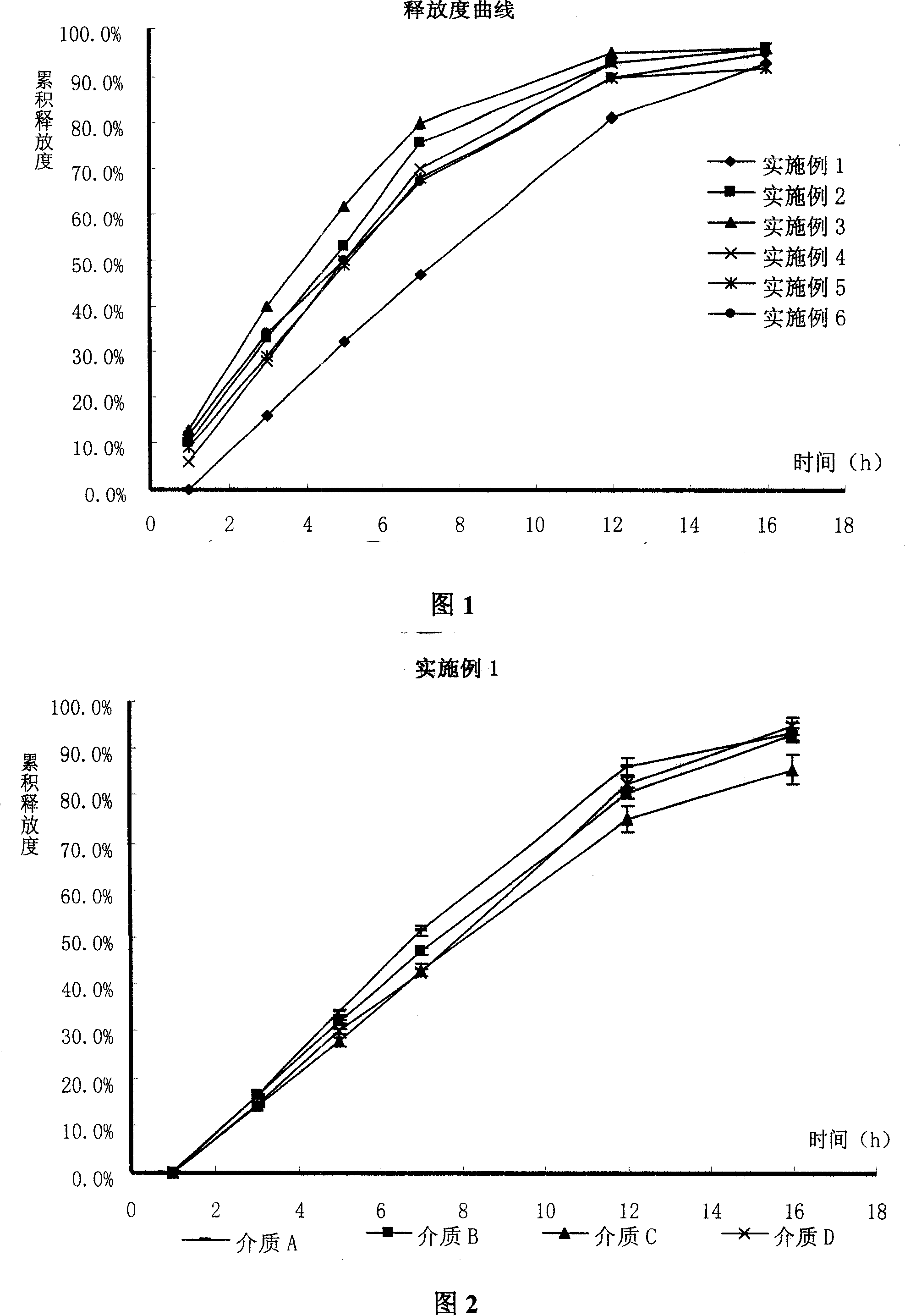
Examples
Embodiment 1-6
[0031] The prescription is as follows:
[0032] prescription
Example 1
Example 2
Example 3
Example 4
Example 5
Example 6
Drug-containing layer
Copovidone
(Plasdone S630)
0.2mg
129mg
0.2mg
100mg
0.8mg
114mg
0.4mg
114mg
0.2mg
89mg
0.2mg
80mg
[0033] Povidone
(Plasdone K90)
Micropowder silica gel
-
-
0.07mg
1mg
0.5mg
-
-
0.07mg
1mg
0.5mg
-
-
0.07mg
1mg
0.5mg
-
-
0.07mg
1mg
0.5mg
-
40mg
0.07mg
1mg
0.5mg
34mg
-
0.07mg
1mg
0.5mg
The total weight of dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com