Mouse immune state monitoring reagent and its preparing process
A reagent and state technology, applied in biological testing, material inspection products, instruments, etc., can solve the problems of affecting the judgment of results, low sensitivity, damage, etc., and achieve comprehensive and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
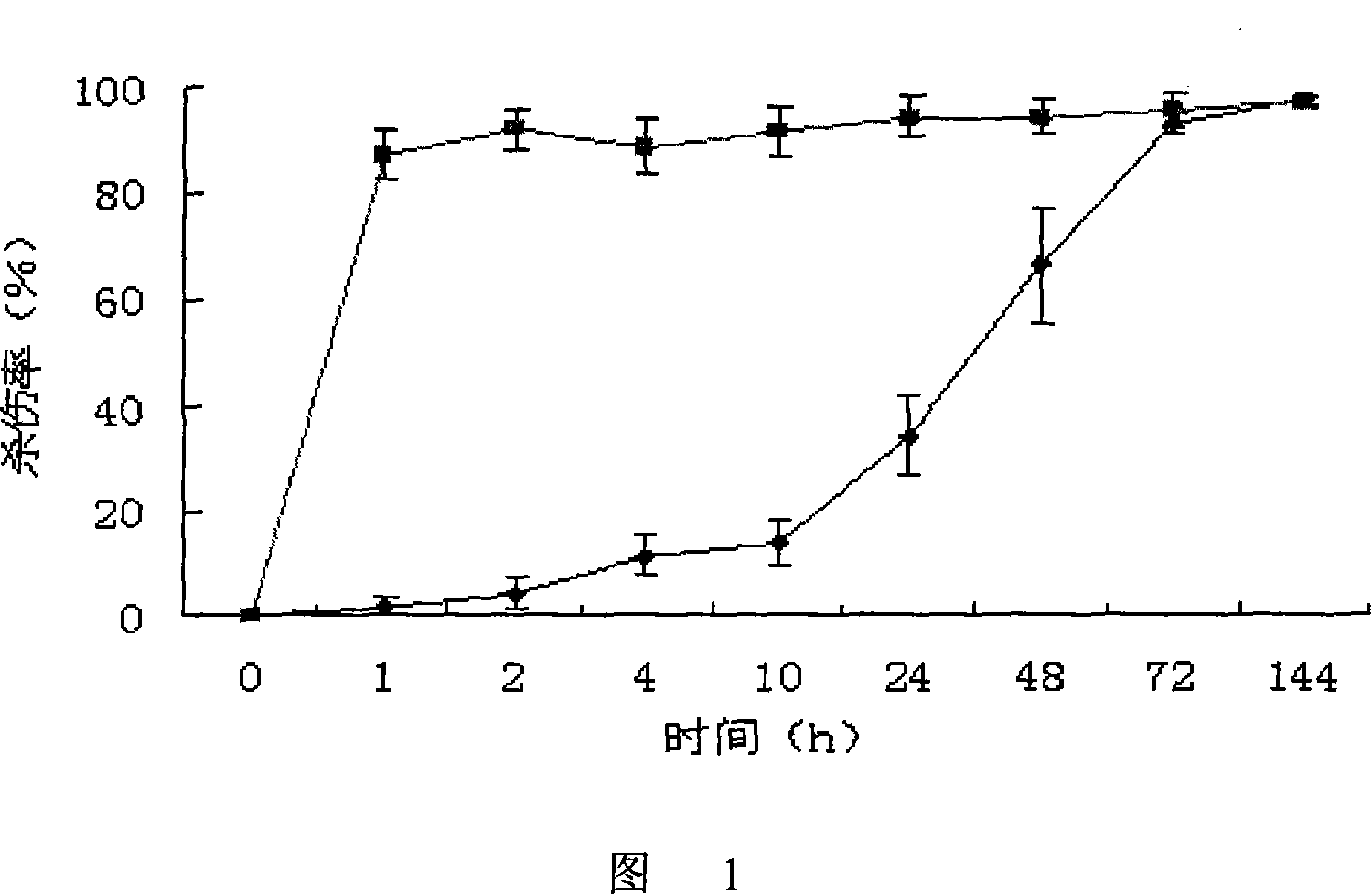
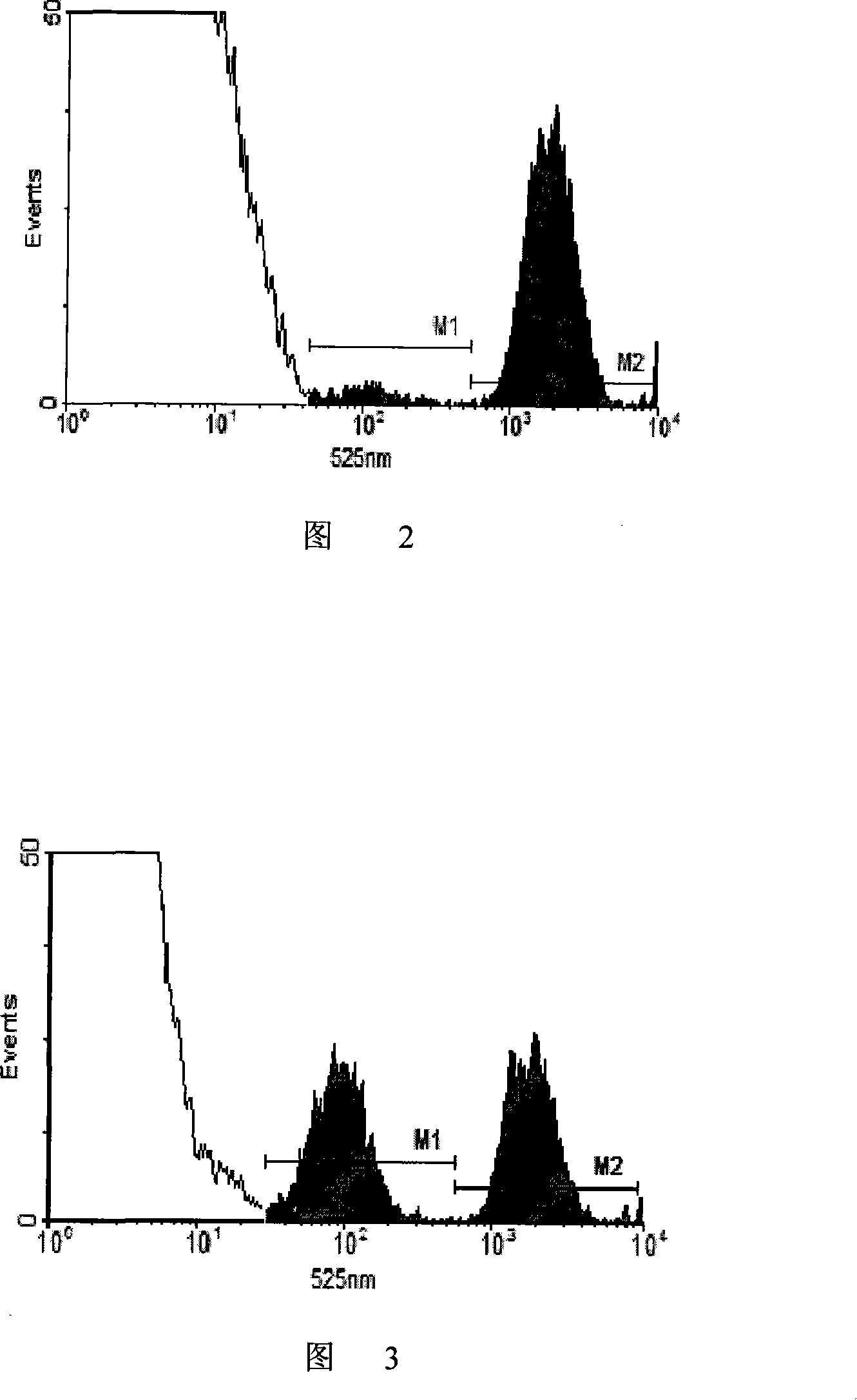
[0138] 1) Take splenocytes from C57 mice and BALB / c mice, suspend them in PBS and adjust the cell concentration to 107 cells / mL;
[0139] 2) Dilute the dimethyl sulfoxide solution of CFSE with a concentration of 10 mM with 50 times of PBS to obtain a 200 μM CFSE working solution;
[0140] 3) Add CFSE working solution to the splenocyte suspension of C57 mice for staining so that the final concentration of CFSE is 0.3 μM, add CFSE working solution to the splenocyte suspension of BALB / c mice to make the final concentration of CFSE 6 μM, and then At 37°C, incubate for 15 minutes;
[0141] 4) The stained cells were washed twice with 0.01M 4°C PBS and then resuspended in PBS;
[0142] 5) Mix the two kinds of fluorescently stained cells according to the ratio of cell number 1:1, and adjust the total cell concentration to 5×107 cells / mL.
[0143] Cell viability test: After diluting an appropriate amount of cell suspension, take a drop of the diluted solution and a drop of 0.5% trypa...
example 2
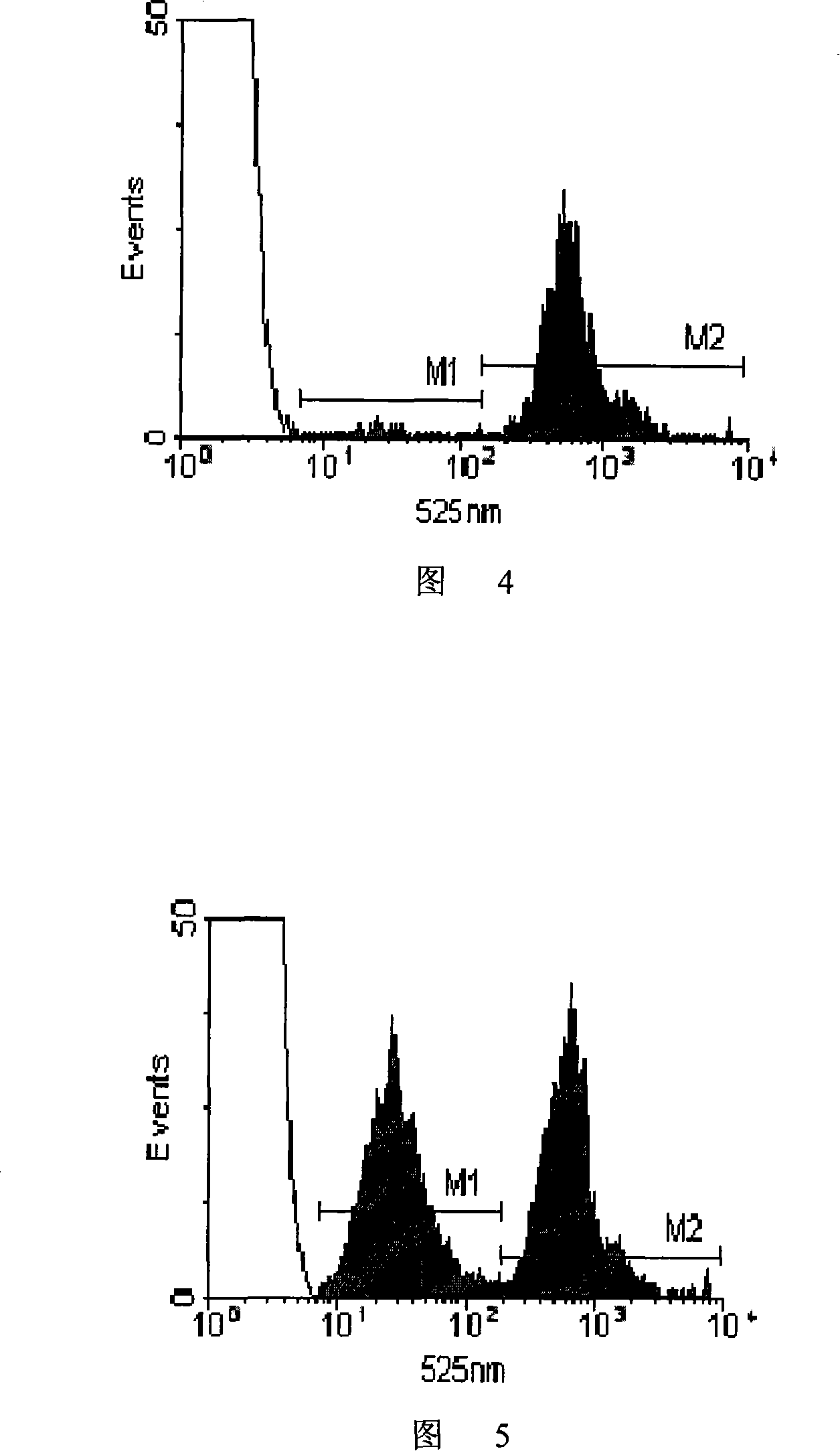
[0147] 1) Take splenocytes from C57 mice and BALB / c mice, suspend them in PBS and adjust the cell concentration to 107 cells / mL;
[0148] 2) Dilute the dimethyl sulfoxide solution of CFSE with a concentration of 10 mM with 50 times of PBS to obtain a 200 μM CFSE working solution;
[0149] 3) Add CFSE working solution to the splenocyte suspension of C57 mice for staining so that the final concentration of CFSE is 0.3 μM, add CFSE working solution to the splenocyte suspension of BALB / c mice to make the final concentration of CFSE 6 μM, and then At 37°C, incubate for 17 minutes;
[0150] 4) The stained cells were washed 3 times with 0.01M 4°C PBS and then resuspended in PBS;
[0151] 5) Mix the two kinds of fluorescently stained cells according to the ratio of cell number 1:1, and adjust the total cell concentration to 8×107 cells / mL.
example 3
[0153] 1) Take splenocytes from C57 mice and BALB / c mice, suspend them in PBS and adjust the cell concentration to 107 cells / mL;
[0154] 2) Dilute the dimethyl sulfoxide solution of CFSE with a concentration of 10 mM with 50 times of PBS to obtain a 200 μM CFSE working solution;
[0155] 3) Add CFSE working solution to the splenocyte suspension of C57 mice for staining so that the final concentration of CFSE is 0.3 μM, add CFSE working solution to the splenocyte suspension of BALB / c mice to make the final concentration of CFSE 6 μM, and then At 37°C, incubate for 20 minutes;
[0156] 4) The stained cells were washed 3 times with 0.01M 4°C PBS and then resuspended in PBS;
[0157] 5) Mix the two kinds of fluorescently stained cells according to the ratio of cell number 1:1, and adjust the total cell concentration to 1×108 cells / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com