Method of producing high purity clofarabine
A high-purity technology for clofarabine, which is applied in the field of preparation of clofarabine, can solve the problems that clofarabine cannot be prepared economically and effectively, the refined yield is only 31.6%, and it is difficult to effectively separate, so as to achieve low cost and reduce impurities content, the effect of economic production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
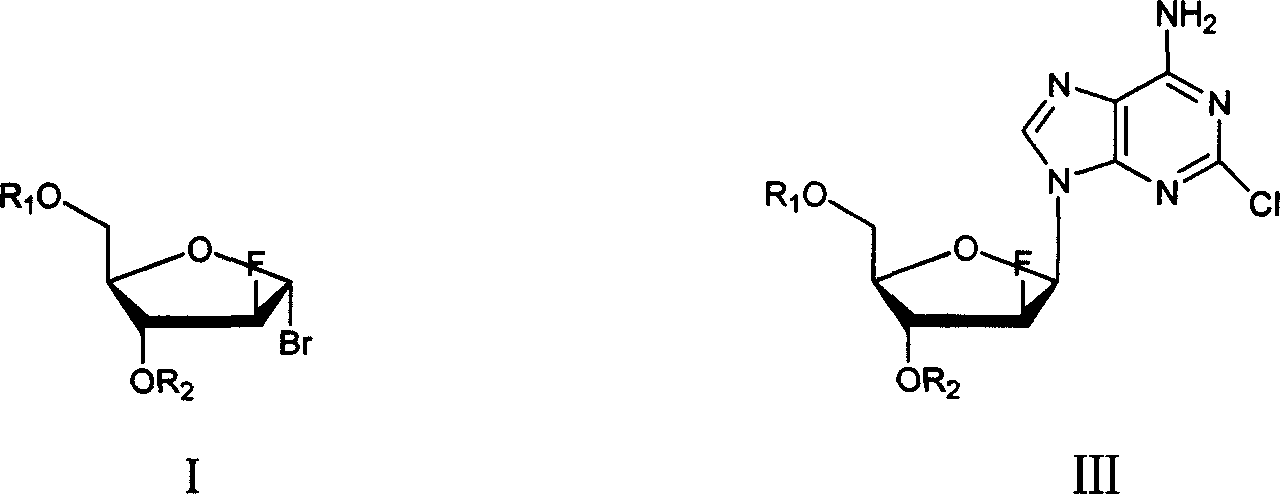
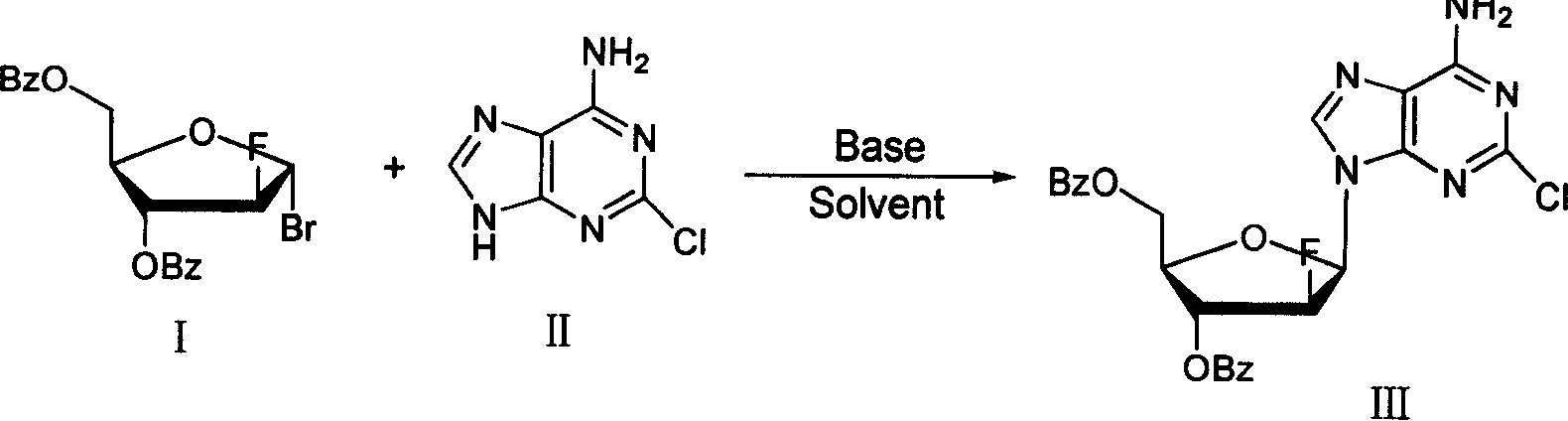
[0026] Under the protection of nitrogen, 12.9g of 2-chloroadenine, 100ml of isopropanol and 75ml of acetonitrile were added into a 500ml three-neck flask, and stirred evenly. At room temperature, 3.2 g of sodium methoxide and 3.4 g of calcium hydride were added. A mixture of 100ml of chloroform and 30.5g of the compound of formula I was slowly added dropwise. The reaction was carried out at room temperature overnight, and TLC detected that the reaction was complete. Filter and wash the filter cake with chloroform. The organic phases were combined and dried over anhydrous sodium sulfate. Suction filtration, and the filtrate was concentrated under reduced pressure. Cooling and crystallization, suction filtration, and vacuum drying at room temperature yielded 27.6 g of a solid (compound of formula III). Yield 75%.
Embodiment 2
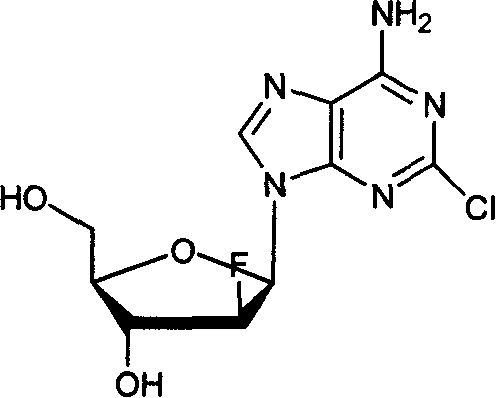
[0028] Add 18.8g of the compound of formula III obtained in Example 1, 3.4g of lithium hydroxide, 200ml of acetonitrile and 350ml of water into a 1L reaction flask. Stir to mix well, and stir at room temperature for 3 hours. Adjust the pH to 7-8 with acetic acid, concentrate under reduced pressure to remove acetonitrile, and cool the residue to crystallize. Suction filtration, the filter cake is beaten with acetonitrile. Suction filtration, and the filter cake was vacuum-dried at 50°C until constant weight. 7.2 g of off-white solid was obtained, the yield was 65%. HPLC analysis did not detect the "α-isomer" of clofarabine.
Embodiment 3
[0030] Under the protection of nitrogen, 12.9g of 2-chloroadenine, 100ml of isopropanol and 50ml of acetonitrile were added into a 500ml three-necked flask, and stirred evenly. At room temperature, 3.2 g of sodium methoxide and 3.4 g of calcium hydride were added. A mixture of 130ml of chloroform and 30.5g of the compound of formula I was slowly added dropwise. The reaction was carried out at room temperature overnight, and TLC detected that the reaction was complete. Filter and wash the filter cake with chloroform. The organic phases were combined and dried over anhydrous sodium sulfate. Suction filtration, and the filtrate was concentrated under reduced pressure. Cooling and crystallization, suction filtration, and vacuum drying at room temperature yielded 27.6 g of a solid (compound of formula III). Yield 70%. According to the method hydrolysis in embodiment 2 again, the product obtained is analyzed by HPLC, and the content of the "α-type isomer of clofarabine is 2.0%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com