Method for purifying human papilloma virus advanced protein from prokaryote
A papilloma and virus technology, applied in the field of purifying human papilloma virus late protein L1 from prokaryotes, can solve the problems of large protein loss, inability to apply large-scale production, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0159] The expression systems currently used in the preparation of HPV VLPs can be divided into eukaryotic expression systems and prokaryotic expression systems.
[0160] The natural conformation of the HPVL1 protein expressed in the eukaryotic expression system is less disrupted, and can spontaneously form VLPs, often requiring only a simple purification process to obtain VLPs with the correct conformation. However, the baculovirus expression system and yeast expression system currently used in eukaryotic expression systems have defects such as low expression levels and high culture costs, which have brought great difficulties to large-scale industrial production.
[0161] Among the prokaryotic expression systems, the Escherichia coli expression system has the advantages of low culture cost and large expression amount. However, the HPVL1 protein expressed in the E. coli expression system often loses its correct natural conformation and is expressed in the precipitate in the f...
Embodiment 1
[0165]Example 1: Construction of recombinant HPV16L1 gene expression vector and expression of HPV16L1 protein
[0166] Construction of pTO-T7-HPV16L1 non-fusion expression vector
[0167] Using DNA extracted from vaginal secretions of cervical cancer patients in Xiamen, Fujian Province as a template, 16H5521F: 5'-TAT AgT TCC Agg gTC TCC AC-3' (SEQ ID NO: 1) as a forward primer, 16H7190R: 5 '-ACA ACA AAC AAC ACT AAT TCA A-3' (SEQID NO: 2) is a reverse primer, and the PCR reaction is carried out in a PCR thermal cycler (T3 type PCR instrument produced by Biometra Company) according to the following conditions: 94°C for 5 minutes ; followed by 25 cycles of 94°C for 50 seconds, 57°C for 50 seconds, and 72°C for 2 minutes and 30 seconds; and finally an extension at 72°C for 10 minutes. A specific product with a size of about 1.6kb was obtained, which was used as a template for another PCR reaction. c16HL5653F: 5'- ggA TCC CAT ATg CTT CCT AgT gAg gCC ACT gTC-3' (SEQ ID NO: 3)...
Embodiment 2
[0175] Example 2 Study on Salt-Free Precipitation and Reconstitution Conditions of HPV16L1 Protein
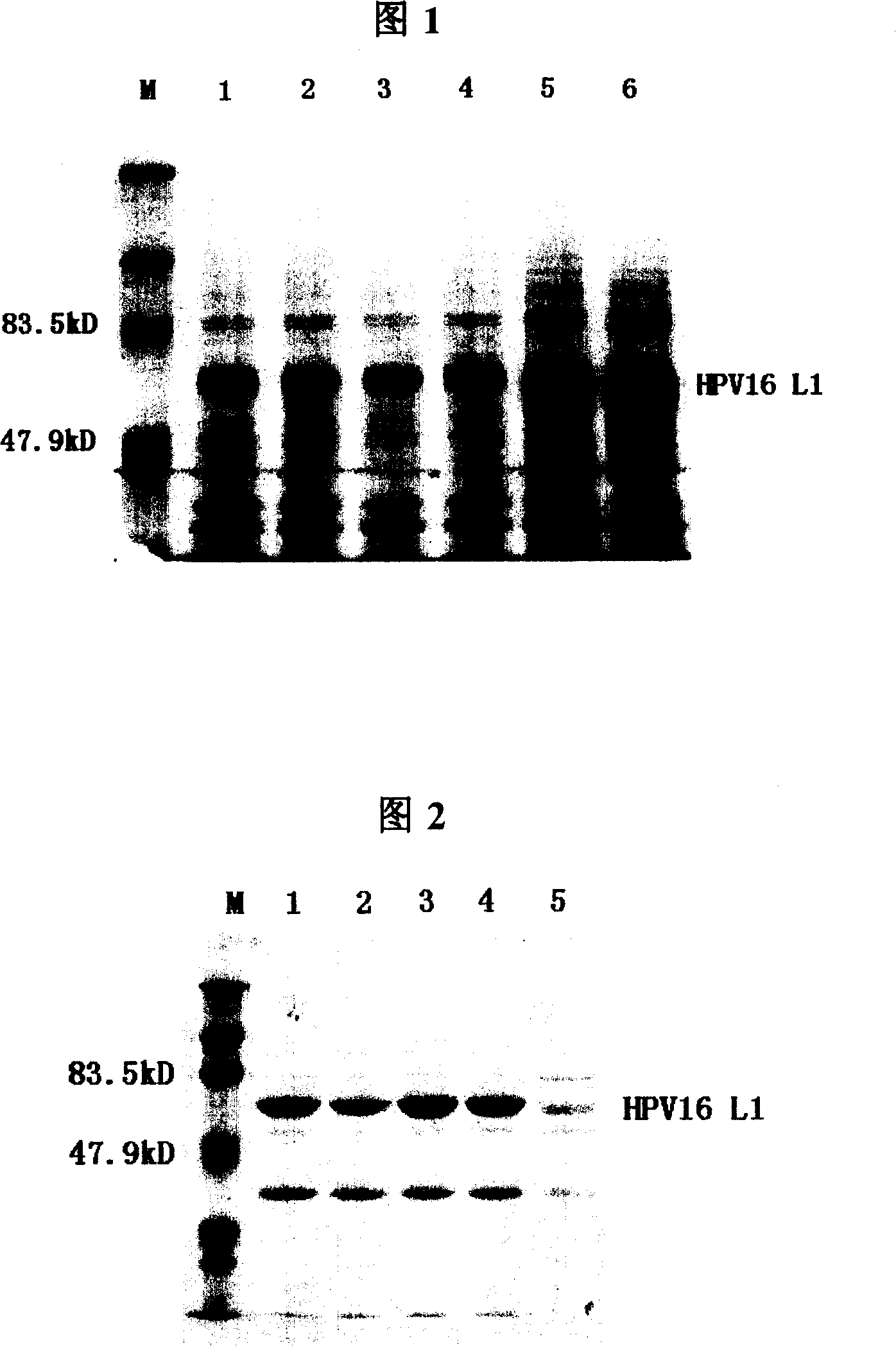
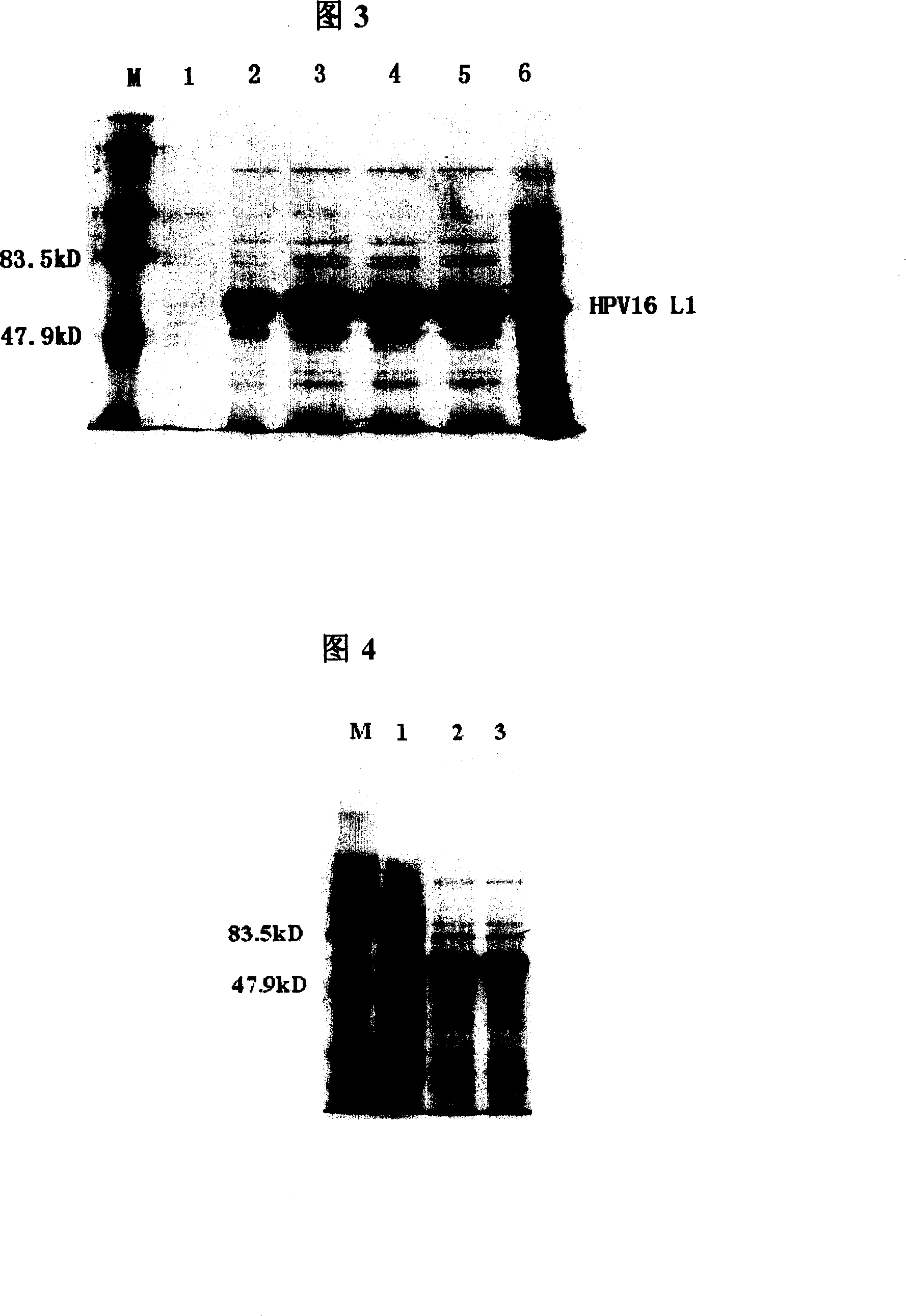
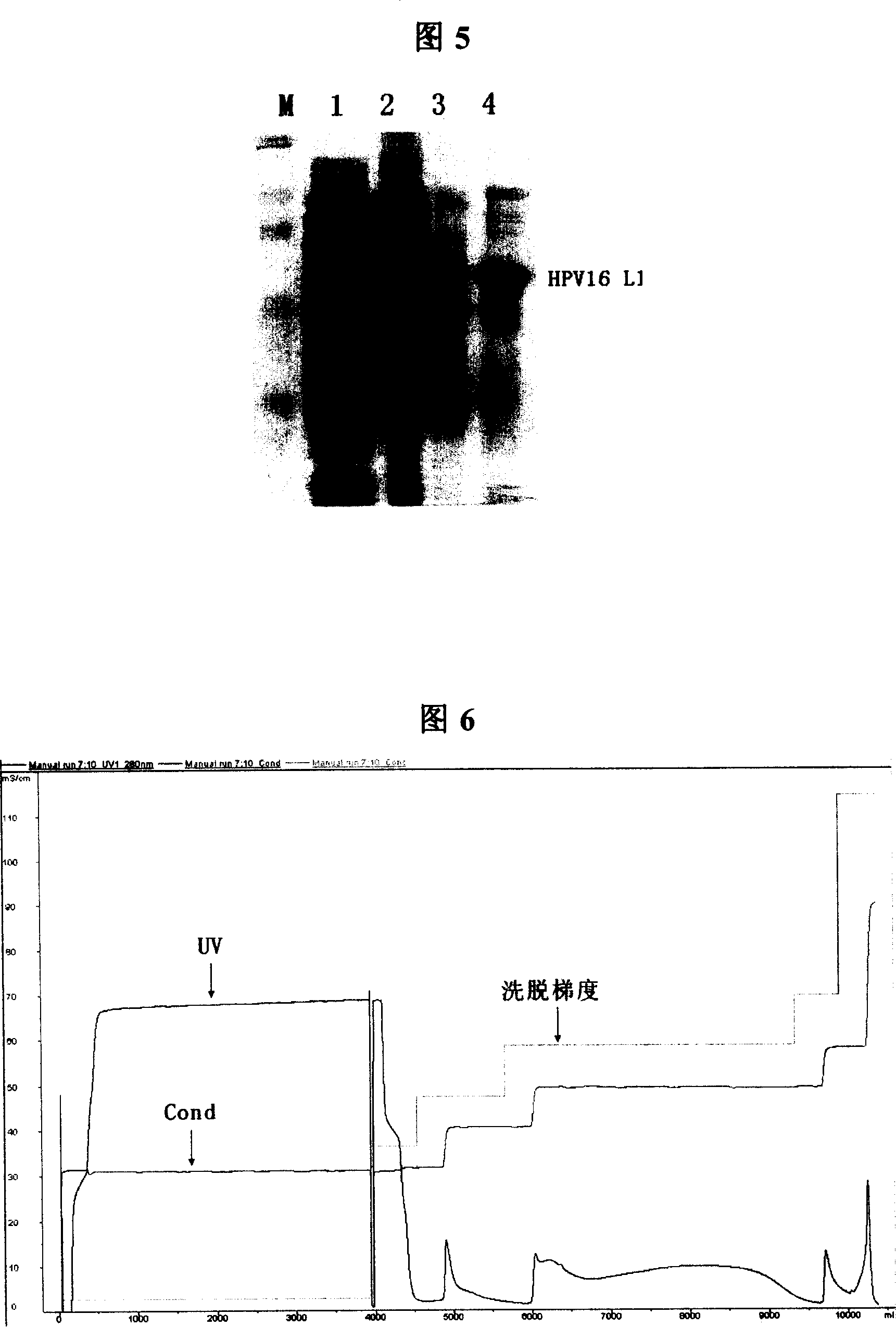
[0176] Massive expression of HPV-16L1 protein
[0177] Each 1L Erlenmeyer flask was filled with 500mL LB liquid medium (kanamycin resistant) to inoculate 100 μL of seed bacteria, shake the flask at 37°C for 8 hours, add IPTG to a final concentration of 0.3mM, and induce at 25°C for 6 hours. The expressed HPV-16L1 protein is expressed in the cytoplasm in two forms of inclusion body and soluble protein, and the protein expressed in the soluble form can be released into the cell lysate supernatant after the cells are broken. Although the relative content of soluble protein is low, it can still be purified by the specific salt-free precipitation method adopted in the present invention.
[0178] Determination of cell lysate
[0179] Centrifuge at 8500rpm for 10min to collect the bacteria. The precipitate obtained by centrifuging 1000mL of bacterial liquid was resuspended with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com