Method for preparing camptothecin medicine slow releasing microcapsule
A technology for slow-release microcapsules and camptothecin, which is applied in the directions of drug combination, drug delivery, and pharmaceutical formulation to achieve the effects of environmental friendliness, high toxicity and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Disperse the camptothecin microcrystals in deionized water, and ultrasonically disperse them for 50 minutes to obtain a suspension of the camptothecin microcrystals,
[0030] (2) Place camptothecin microcrystals in 50 mg / mL chitosan (CHI) solution for adsorption for 30 minutes, centrifuge to remove unadsorbed chitosan (CHI), and repeat washing with 1 mmol / L hydrochloric acid solution for 10 minutes times, each washing for 10 minutes,
[0031] (3) Place camptothecin microcrystals in 50 mg / mL sodium alginate (ALG) solution, adsorb for 30 minutes, centrifuge to remove unadsorbed sodium alginate (ALG), and wash repeatedly with 1 mmol / L hydrochloric acid solution 10 times, 10 minutes each wash,
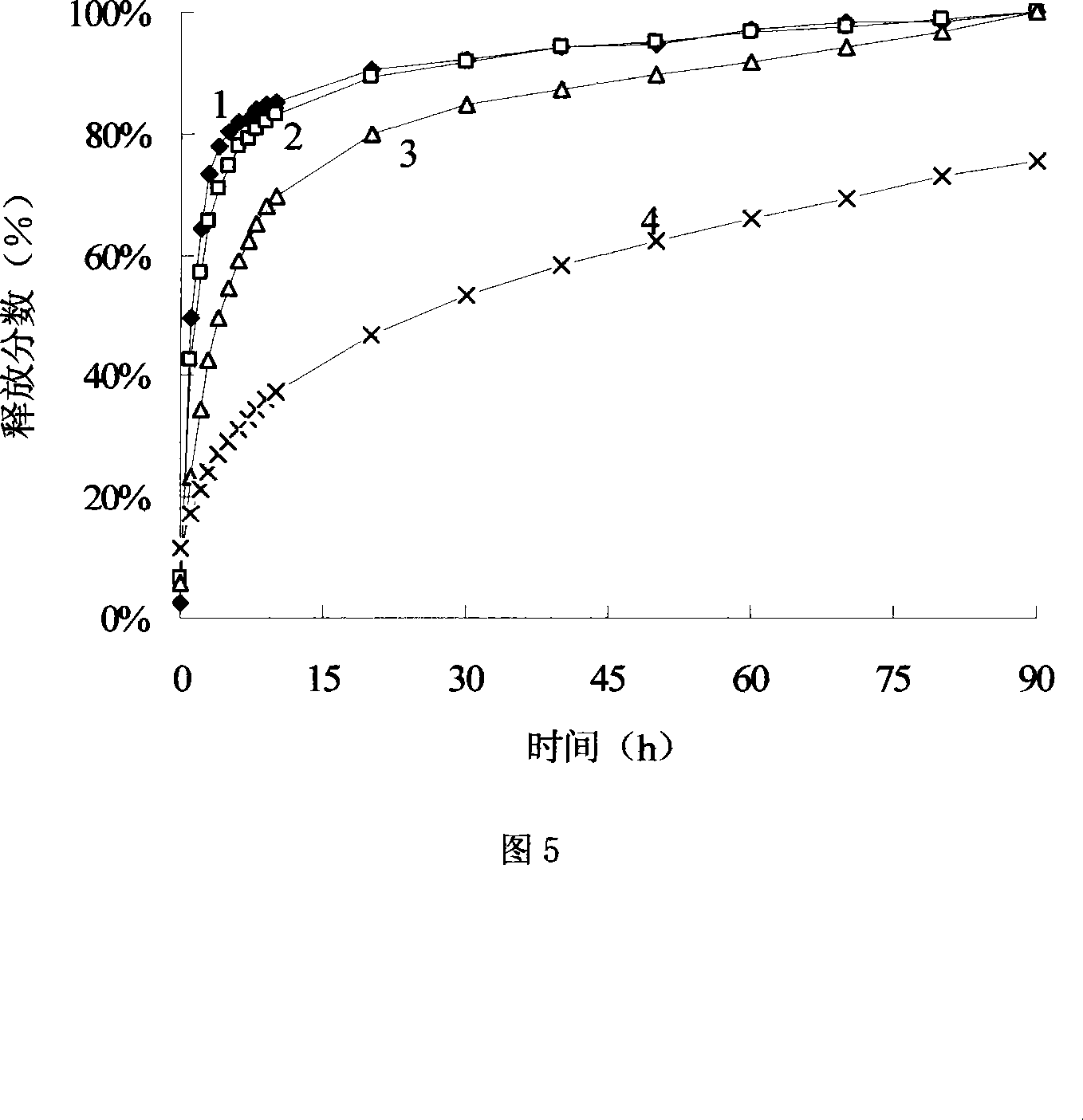
[0032] (4) repeat step (2) and (3) successively, until obtaining the camptothecin microcapsules of capsule wall layer respectively 5,11,17,
[0033] (5) Place the camptothecin microcapsules coated with 5 layers of polymers under a laser confocal scanning electron microscope to...
Embodiment 2
[0040] (1) 10-hydroxycamptothecin microcrystals were dispersed in deionized water, ultrasonically dispersed for 50 minutes to obtain a suspension of 10-hydroxycamptothecin microcrystals,
[0041] (2) 10-Hydroxycamptothecin microcrystals were placed in 50mg / mL ferric chloride solution with pH=2 to absorb for 30 minutes, centrifuged to remove unadsorbed ferric chloride, and washed repeatedly with 1mmol / L hydrochloric acid solution for 10 minutes times, each washing for 10 minutes,
[0042](3) Place 10-hydroxycamptothecin microcrystals in 50 mg / mL dextran sulfate (DS) solution, and absorb for 30 minutes; centrifuge to remove unadsorbed DS, and wash repeatedly with 1 mmol / L hydrochloric acid solution for 10 times, each washing for 10 minutes,
[0043] (4) repeat step (2) and (3) successively, until obtaining the 10-hydroxycamptothecin microcapsules that capsule wall layer number is respectively 8,12,16,
[0044] (5) Dissolve the 10-hydroxycamptothecin in the microcapsules with d...
Embodiment 3
[0049] (1) 9-nitrocamptothecin microcrystals were dispersed in deionized water, and ultrasonically dispersed for 50 minutes; the suspension of 9-nitrocamptothecin microcrystals was obtained,
[0050] (2) 9-nitrocamptothecin microcrystals were placed in a 50 mg / mL pH=2 polyallyl ammonium hydrochloride (PAH) solution for 30 minutes, centrifuged to remove unadsorbed PAH, and 1 mmol / L hydrochloric acid solution was washed 10 times, each time for 10 minutes,
[0051] (3) Put the 9-nitroxime microcrystals in 50 mg / mL polystyrene sodium sulfonate (PSS) solution, adsorb for 30 minutes, centrifuge to remove unadsorbed PSS, and wash repeatedly with 1 mmol / L hydrochloric acid solution 10 times, 10 minutes each wash,
[0052] (4) Steps (2) and (3) were repeated successively until the camptothecin microcapsules with capsule wall layers of 3 and 5 were obtained.
[0053] In the present embodiment, polycation can also select chitosan, protamine, polyarginine, polydiallyldimethyl quaternar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com