Electrochemistry detecting method and testing apparatus of saccharification hemoglobin content
A technology of glycosylated hemoglobin and a detection method, which is applied in the field of determination of glycosylated hemoglobin in blood, can solve the problems of expensive instruments and reagents, cumbersome operation steps, and long detection cycle, and achieve the effects of low cost, simple and flexible operation, and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Preparation of glycosylated hemoglobin recognizer
[0015] (1) Glass balls with a particle size of 5-10 microns are first activated with 0.1mol / L sodium hydroxide solution for 1 hour, washed with water, then added to 10mmol / L trimethoxy-solution and soaked for 2 hours to obtain trimethyl Oxysilane modified glass spheres, and introduced -CHO active group on the surface of glass spheres. After washing and centrifuging, soak the modified glass beads in 10mmol / L plant lectin ConA (pH 7.4) or aminophenylboronic acid solution for 5 hours, and wash 3 times with pH 7.4 phosphate buffer solution to obtain Plant lectin ConA or aminophenylboronic acid modify the glycosylated hemoglobin recognition medium, fill it into a glass tube, and obtain the glycosylated hemoglobin separation column after solidification.
[0016] (2) According to Si: H 2 O molar ratio 1:4 ratio takes a certain amount of tetraethoxysilane or tetraethoxysilane and the triethoxy-3-silane mixture that accounts ...
Embodiment 2
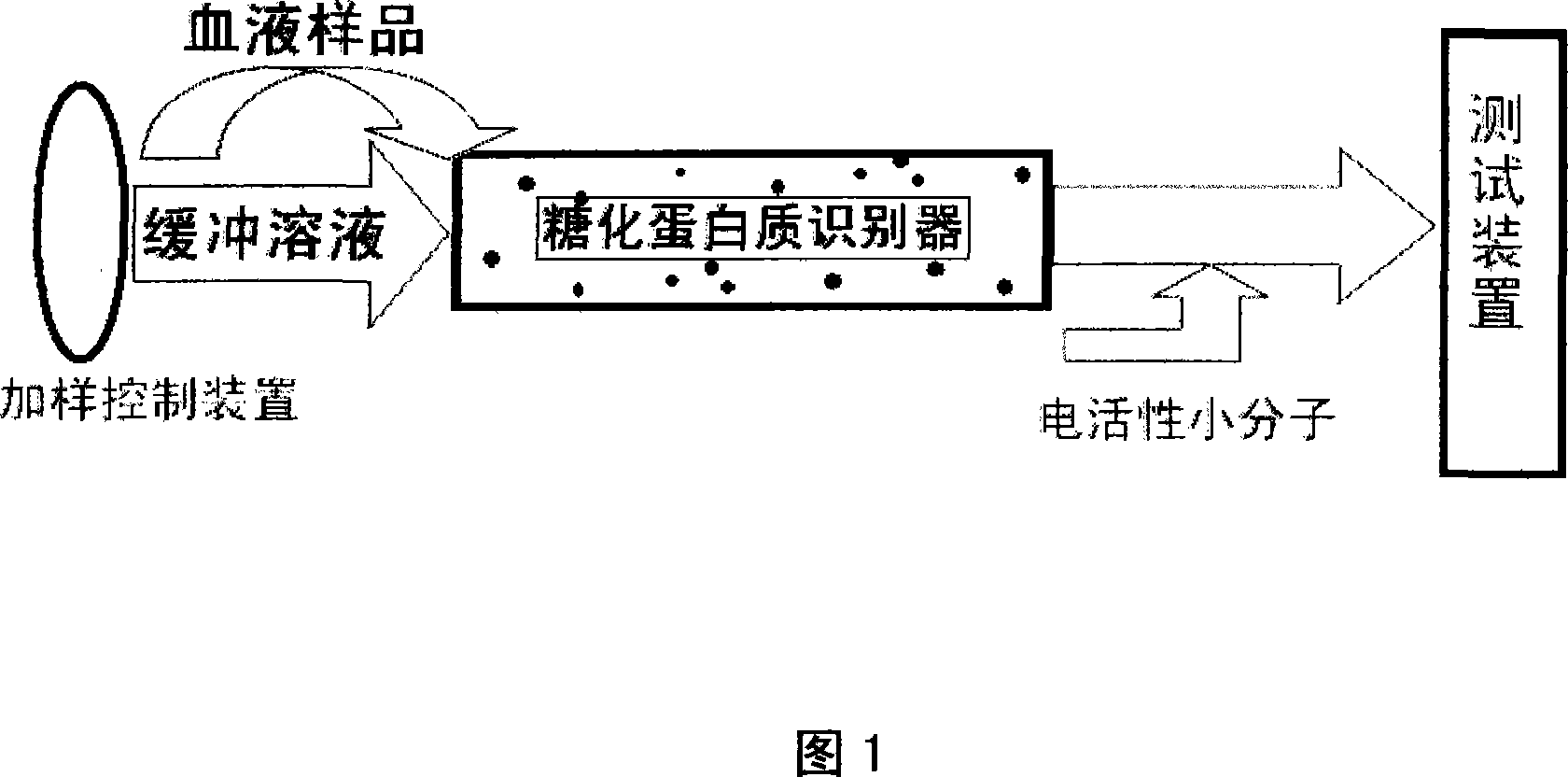
[0038] An electrochemical detection method for glycosylated hemoglobin content, after diluting the whole blood sample 30-70 times with a binding solution, passing the glycosylated protein identifier, adding electroactive small molecules to the effluent of the whole blood sample of the glycosylated protein identifier, and using The chemically modified electrode measures the electrochemical response of the post-column solution to obtain the concentration a of hemoglobin; the glycosylated protein identifier is eluted with the eluent, and the electrochemical response of the eluent is measured to obtain the concentration b of the glycosylated hemoglobin, and b / (b+a) Obtain the content of glycated hemoglobin in the sample. The glycosylated hemoglobin recognizer uses plant lectin ConA or aminophenylboronic acid modified glass beads, silane hybrid sol or agarose gel as medium. The binding solution is a Heps buffer solution with a pH value of 7.0-8.5. The chemically modified electrod...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com