Preparation and composition of inter-alpha inhibitor proteins from human plasma for therapeutic use
A technology of alpha inhibitors and compositions, applied in the fields of inhibiting metastasis and treating sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
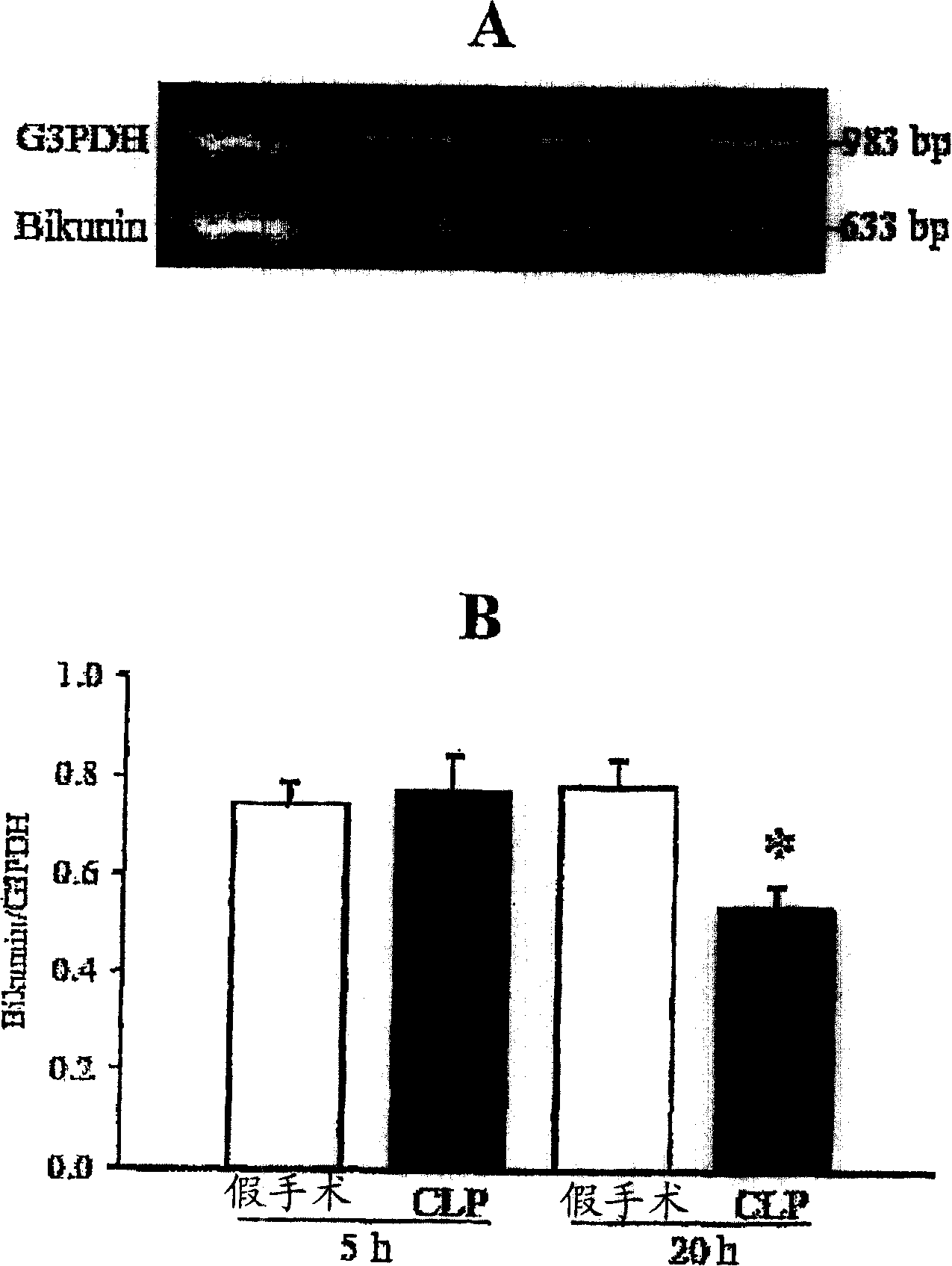
[0123] Characterization of H4 as part of the IαIp complex
[0124] SELDI-TOF mass spectrometry analysis indicated that heavy chain 4 (H4) was also present in a 125 kDa band (PαI) and a 250 kDa band (IαI) (data not shown). The presence of H4 is more pronounced in the 250 kDa band. This indicates that another complex protein other than IαI (H1+H2+LC) and PαI (H3+LC) may be present in some compositions of the invention. To date, no complex form of H4 has been described. Free H4 is less than 125 or 250 kDa. Due to the mass spectrometry results, we thought that H4 might be complexed with bikunin (light chain) or others.
Embodiment 2
[0126] Animal Models of Sepsis
[0127] Male Sprague-Dawley rats (275-325 g) were housed in a temperature-controlled room on a 12-h light / dark cycle and fed a standard Purina rat solid diet. Before the induction of sepsis, rats were fasted overnight but had access to water ad libitum. Rats were anesthetized with isoflurane inhalation, the ventral neck, abdomen and groin were shaved and washed with 10% povidone-iodine. A 2-cm midline laparotomy was performed. The cecum is exposed, ligated at the end of the ileocecal valve to avoid ileus, punctured twice with an 18-gauge needle, and gently squeezed to allow a small amount of fecal matter to flow out of the hole and return to the abdominal cavity; the abdominal incision is then sutured layer by layer . Sham-operated animals (ie, control animals) were subjected to the same procedure except that the cecum was neither ligated nor punctured. Animals were revived immediately after surgery with subcutaneous 3ml / 100gBW saline. Anim...
Embodiment 3
[0150] Purification of IαIp
[0151] After applying dialysis or ultrafiltration / diafiltration, the eluate was extracted with DEAE Sephadex A50 solid phase, and the pH6.0 containing 0.28M sodium chloride 0.005M sodium citrate / 0.0055M sodium phosphate buffer was used The column elutes weakly bound components (described in Hoffer et al., Journal of Chromatography B 669 (1995) 187-196). In the previous step, the column was washed with 0.005M sodium citrate / 0.0055M sodium phosphate buffer containing 0.20M sodium chloride at pH 6.0.
[0152] After dialysis or ultrafiltration / diafiltration (UF / DF) against 0.005M sodium phosphate buffer at pH 7.0, the eluate was applied to a hydroxyapatite column. IαIp does not bind to the column and is collected as the effluent fraction. Contaminant proteins, mainly FII, FVII, and FX, can be eluted using a gradient of increasing concentrations of sodium phosphate buffer. IαI / PαI contains more than 90% of the target protein, mainly IαI and PαI.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com