Composition for adjuvant containing poly-gamma-glutamic acid
A technology of synthetic and glutamic acid, applied in the local field, can solve problems such as being unsuitable for mass production, difficult to decompose vaccines in the body, and easily affected in quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Production of poly-γ-glutamic acid
[0032] Containing 3L basic medium (GS medium containing 5% L-glutamate, 5% glucose, 1% (NH 4 ) 2 SO 4 , 0.27% KH 2 PO 4 , 0.42% Na 2 HPO 4 .12H 2 O, 0.05% NaCl, 0.3% MgSO 4 .7H 2 (0, 1ml / L vitamin solution, pH 6.8) was inoculated with 1% Bacillus subtilis var.chungkookjang (KCTC 0697BP) culture solution in a 5L fermenter, and cultivated at a stirring speed of 150rpm, an air blowing rate of 1vvm and 37°C After 72 hours, 2N sulfuric acid solution was added to adjust the pH to 3.0, thereby obtaining a sample solution containing poly-γ-glutamic acid.
[0033] The sample solution was left standing at 4°C for 10 hours to remove polysaccharides present in the fermentation broth, and two volumes of ethanol was added to the fermentation broth, followed by thorough mixing. The mixed solution was left to stand at 4°C for 10 hours, and then centrifuged to obtain a precipitate of poly-γ-glutamic acid. The precipitate was d...
Embodiment 2
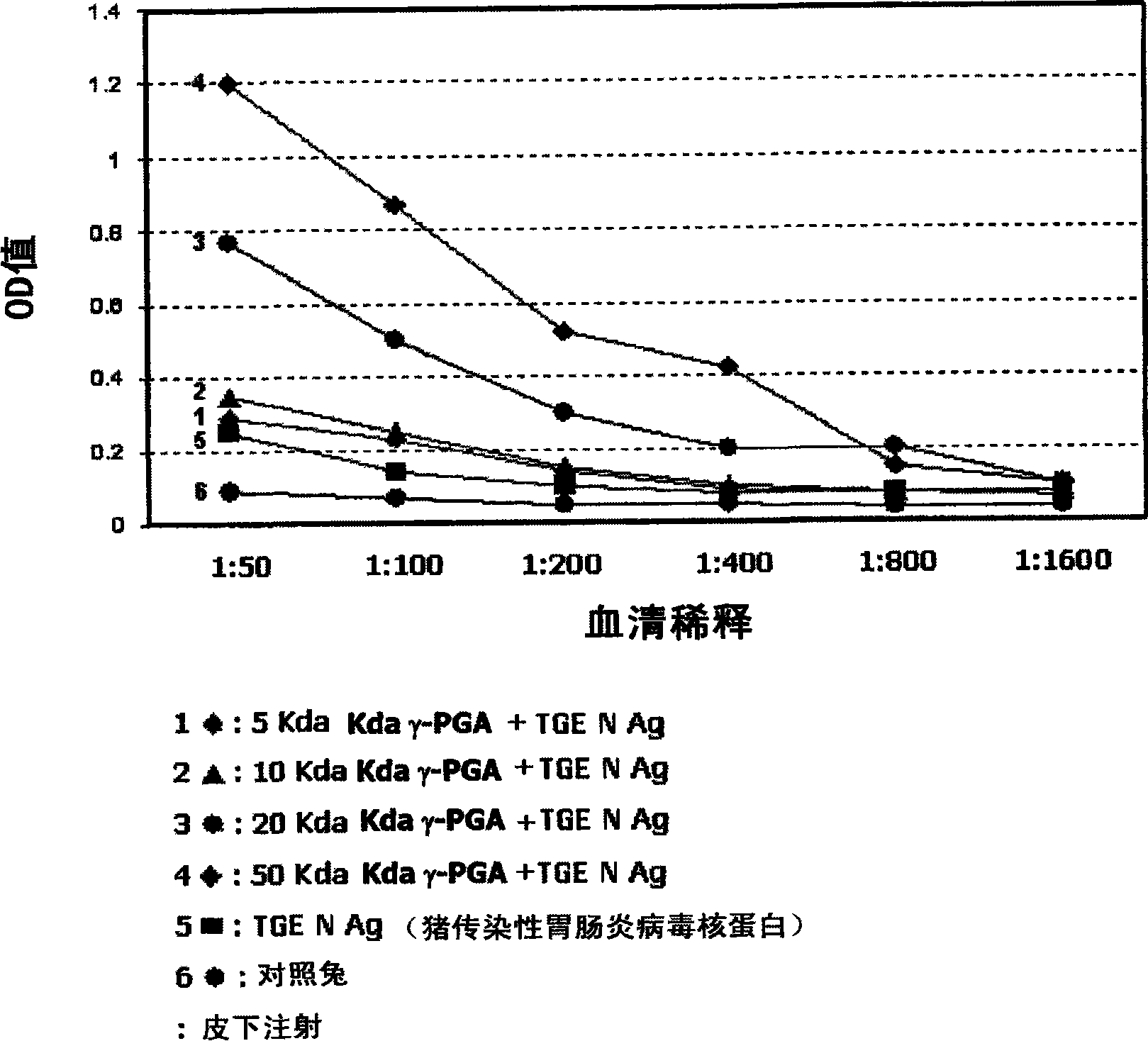
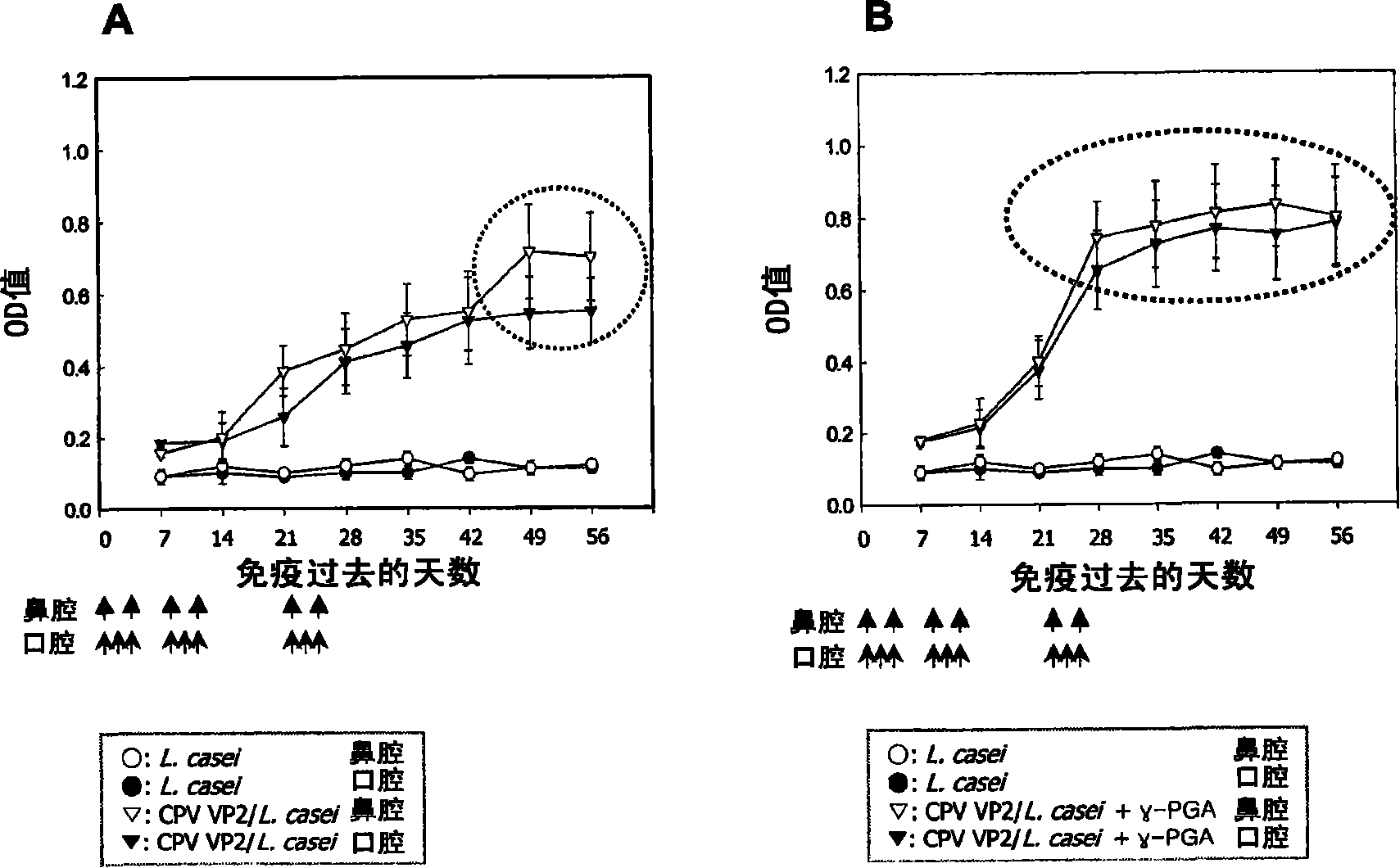
[0035] Example 2. Production of antibodies against TGE viral antigens by poly-γ-glutamic acid
[0036] In this example, in order to examine whether the inventive poly-γ-glutamic acid exhibits an immunoenhancing effect specific to a soluble antigen, in the specific immune response to the antibody, in particular, it was examined against the B involved in the production of the antibody. The effect of the humoral immune response elicited by cells. Nucleoprotein (N) of porcine transmissible gastroenteritis virus (TGE), which induces porcine transmissible digestive organ disease, was used as an antigen, and rabbits were used as test animals.
[0037] Rabbits subcutaneously injected with only TGEN antigen (400μg / PBS) were used as a control group, TGEN antigen (400μg / PBS) and poly-γ-glutamic acid with molecular weights of 5kDa, 10kDa, 20kDa, and 50kDa were mixed and injected subcutaneously Rabbits were used as the experimental group.
[0038] Two weeks after the first subcutaneous...
Embodiment 3
[0041] Example 3. Production of antibodies against HBV virus by poly-γ-glutamic acid
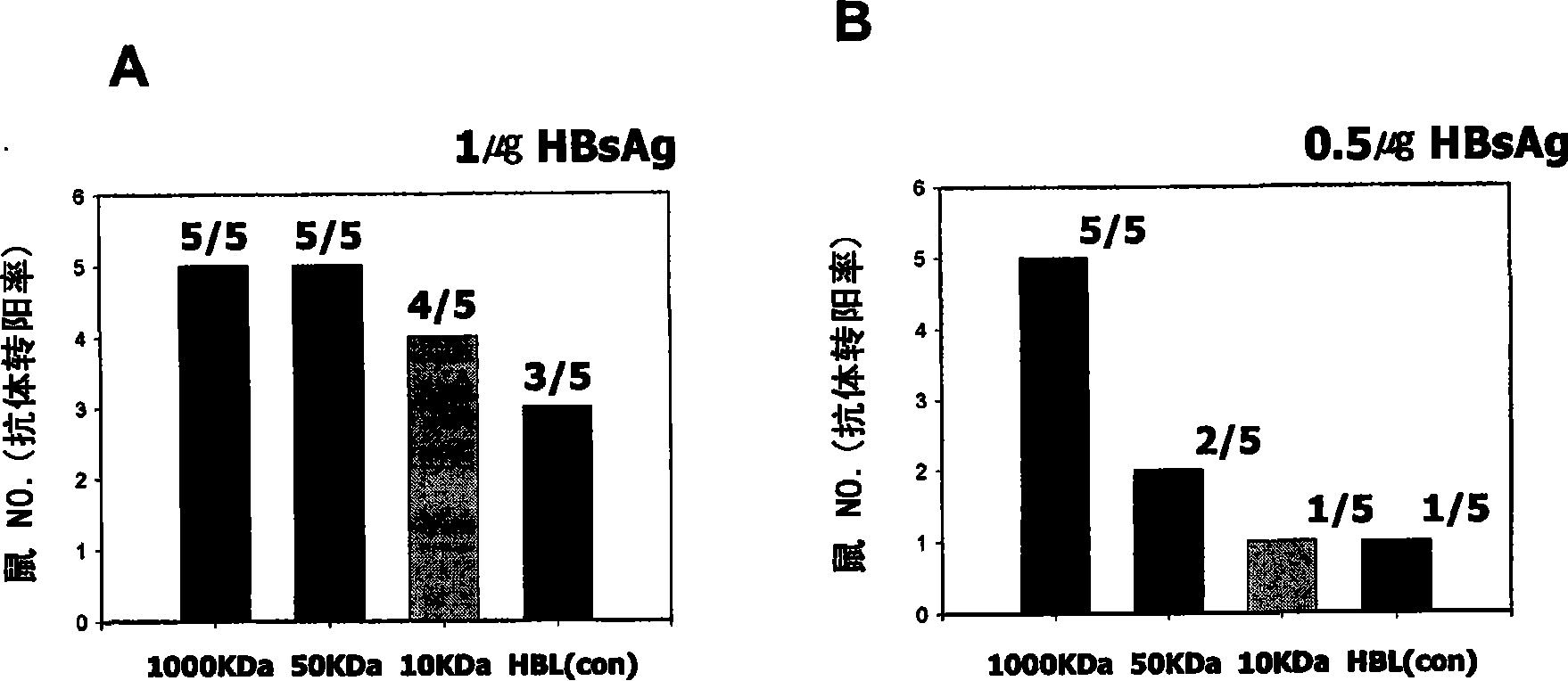
[0042] In the examples, in order to examine whether poly-γ-glutamic acid exhibits specific immune enhancement effects (humoral immune response) on other soluble antigens by intraperitoneal injection, Balb / c mice were used as test animals against hepatitis B virus from yeast (HBV) surface antigen test.
[0043] As a control group, 6-week-old Balb / c female mice were injected with purified HBsAg (hepatitis B virus surface antigen) L particle antigen (1 μg / PBS ml) alone in the abdomen, and as a control group, HbsAg L particle antigen (1 μg / PBS ml) ml) and poly-γ-glutamic acid (γ-PGA) with 10 kDa, 50 kDa and 1000 kDa respectively were mixed and injected intraperitoneally. Moreover, with the change of antigen concentration, the control group and HbsAg L particle antigen (0.5 μg / PBS ml) and the molecular weight of 10kDa, 50kDa and A test group in which a poly-γ-glutamic acid mixture of 1000 kDa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com